
What is a Hover Fly and Why are They Important?
Hover flies, also known as flower flies or syrphid flies, are a highly diverse, common, and charismatic group of insects. They can be found in a wide variety of habitats, from forests and prairies to city parks, sports fields, and vacant lots. Most people are likely to have encountered these insects, but few know how to identify them, and even fewer know of the ecological services they provide.
There are over 6,200 species worldwide and 812 in the United States and Canada (Miranda et al., 2013). Most of them are pollinators, predators, and/or decomposers. Most species are also mimics of various bees and wasps, so they are commonly mistaken for stinging insects. However, to the well-trained eye, they can be easily distinguished.
The goal of this publication is to alleviate this “hover fly blindness” by highlighting the importance of these magnificent insects and providing a brief guide to genera and species found in the southeastern United States, specifically the state of Georgia. Users should note that this guide does not cover all 180–190 species known to occur in Georgia, but it does cover those which are most commonly encountered.
Pollination Services
Adult hover flies are almost always anthophilous, meaning that they seek flowers to obtain nectar and pollen for food. Just like bees, they are important in the pollination process for many flowering plants. Pollen picked up from flowers will stick to the fly body, hairs (setae), and legs, where it may be transported from one flower to the next. Pollen that falls off the fly and onto the stigma of a flower can then fertilize that flower and allow it to fruit. Most people do not think of flies when they think of pollinators. In fact, flies as a whole (i.e., members of the taxonomic order Diptera) are widely considered the second-most important group of pollinating insects after bees, and much of this is attributed to hover flies (Orford et al., 2015). In the eastern U.S., hover flies can be important for pollinating a variety of crops including carrots, apples, peaches, mangoes, canola, sweet peppers, onions, and strawberries, among others (Jauker and Wolters, 2008; Rader et al., 2015; Dunn et al., 2020). Many hover flies in Georgia and throughout the Southeast are especially common during spring and fall when temperatures are cooler, making them valuable early- and late-season pollinators. In addition to pollination, there are several other ecosystem services provided by hover flies.
Biological Control and Decomposition Services
Just like many other insects, hover flies exhibit what is called complete metamorphosis. This is where an insect has distinct egg, larval, pupal, and adult stages that all appear and behave completely differently from one another (see Figures 3 and 15). In the case of hover flies, larvae occupy a diverse array of ecological niches that are vastly different from adults and vary by taxonomic grouping.
The most diverse grouping of hover flies, the subfamily Eristalinae, contains species that are essential decomposers, with larvae adapted for dung feeding (coprophagy), fungus feeding (mycophagy), plant feeding (herbivory), and aquatic filter feeding (see Figure 16). In the genus Eristalis, for example, larvae known as rat-tailed maggots (named after their long tail-like breathing siphon), can live in highly putrid conditions like animal waste lagoons and cesspools where they help break down organic waste that may otherwise pollute the environment (Skevington et al., 2019).
Perhaps the best-known hover fly larvae, however, are those of the subfamily Syrphinae, which are mostly terrestrial predators. This subfamily encompasses approximately one-third of all hover fly species and contains some of the most commonly encountered adults like those of the genus Toxomerus (Figure 10). Larvae of these flies resemble tiny green/brown slugs (Figure 4), and are often found on plants where they feed on aphids and other soft-bodied insects, many of which are pests. Damage by aphids to vegetable, grain, and fruit crops causes billions of dollars in losses annually in the United States alone (Bugg et al., 2008; Ragsdale et al., 2011), so these larvae should be considered beneficial to farmers and gardeners. These insects tend to ravage aphid populations (Dunn et al., 2020), with some larvae consuming nearly 1,000 aphids before reaching adulthood (Schneider, 1969), so their presence can eliminate or reduce the need for insecticides. Next time you see an aphid colony, look closely to see if you can find these larvae.
Hover Fly Classification
Taxonomists group all organisms into classifications known as taxa based on morphological, behavioral, and genetic similarities. Hover flies are true flies of the insect order Diptera, specifically grouped into the family Syrphidae. Within the family Syrphidae, the over 200 genera (plural for genus) and 6,200 species of hover flies are all split among four subfamilies based on similarities in morphology and life history traits: Syrphinae, Pipizinae, Eristalinae, and Microdontinae.
These four subfamilies, all of which are found in Georgia, will be discussed in detail throughout this publication.
Distinguishing Features of Hover Flies
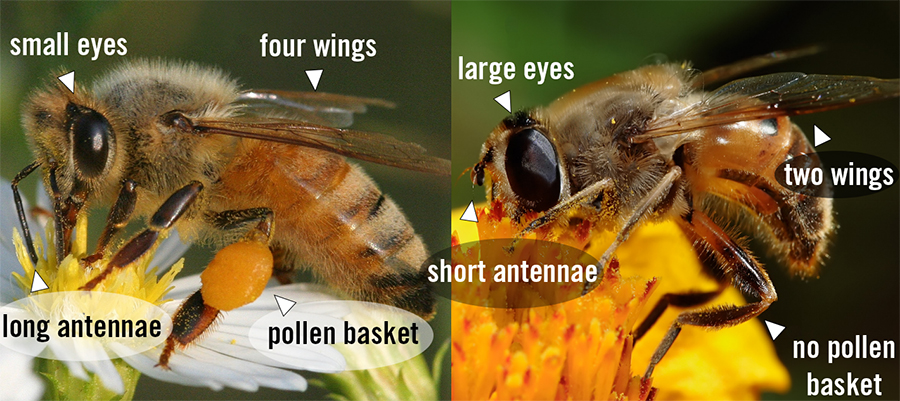
Hover flies are perhaps best known for their tendency to mimic bees and wasps, the purpose of which is to fool predators into thinking that they are harmful or dangerous (Marshall, 2012). Indeed, popular advertisements, news articles, and even scientific publications mistake these flies for bees or wasps. However, hover flies (and other true flies) can be distinguished by having only two wings, short antennae (usually), and large eyes in proportion to the body. Bees and wasps have four wings, longer antennae, and smaller eyes. Additionally, many bees have distinctive pollen baskets that are often packed with orange/yellow pollen.
While hover flies are usually the most common bee- and wasp-mimicking flies, others may be confused with hover flies. For example, some species within the families Asilidae (robber flies), Bombyliidae (bee flies), Conopidae (thick-headed flies), Stratiomyidae (soldier flies), and Tachinidae (parasitic bristle flies) are quite good mimics and may even be spotted visiting flowers. Hover flies can be distinguished from these other groups using several features, but perhaps the clearest feature is the spurious wing vein (Figure 2). This characteristic is found in almost all hover flies except a few rare species.
How to Use This Guide
This guide is intended to assist users in identifying the most common hover flies found in Georgia and throughout much of the southeastern U.S. There are pictures of all common species or genera and arrows pointing to important characteristics. If desired, users can download a PDF of the species group images and names for quick reference.
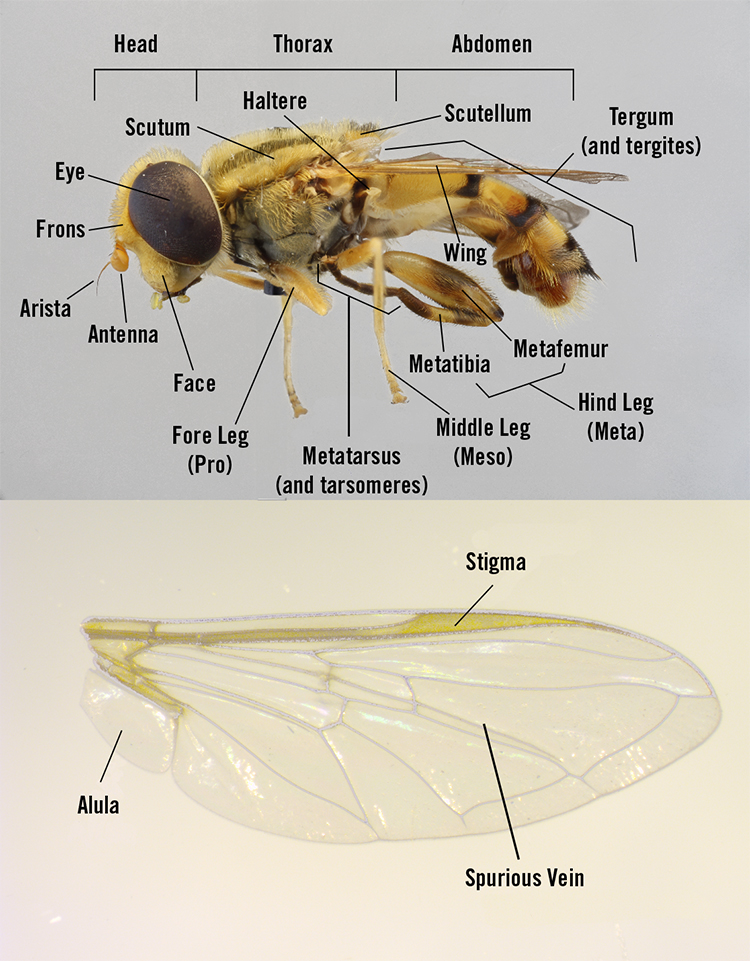
Species are divided into three groups of photographs based on appearance, life history traits (predacious vs. nonpredacious larvae), and subfamily categorization (more information below). For guidance on technical terms related to external morphology, see Figure 2.
Hover Fly Anatomy
Species Photographs
Photographs appear alongside details of each species representing the most common genera and species of hover flies encountered in Georgia. Species have been divided into three groups based on life history and appearance:
- Species with primarily predacious aphid-feeding larvae (Syrphinae and Pipizinae),
- Species with nonpredacious larvae that resemble wasps (Eristalinae part 1)
- Species with nonpredacious larvae that resemble bees (Eristalinae part 2 and Microdontinae).
Exceptions are noted with asterisks (*). If desired, users can download a PDF of the species group images and names for quick reference and comparison. Throughout this guide, readers will see diagrams of general life histories (Figures 3 and 15), photos of representative larvae (Figures 4 and 16), and specific details of each species, including their defining morphological characteristics (also signified by arrows on the photos).
This guide covers the majority of common taxa, but it is not comprehensive and does not include some rare and uncommon taxa that occur in Georgia. There are at least 76 genera of hover flies that occur in Georgia, and this guide covers 32 of those. For simplicity, species that cannot be identified without a microscope are only covered at the genus level. There are currently no comprehensive guides to southeastern Syrphidae, but the Field Guide to the Flower Flies of Northeastern North America (Skevington et al., 2019) provides beautiful details of taxa, many of which also occur in the Southeast. Please see the section at the end of this guide for a list of genera/species that occur in Georgia but are believed to be less-commonly encountered. For an online pictorial key to North American genera, see Miranda et al. (2013).
Species Information
Group 1: Species With Predacious Larvae
Subfamily Syrphinae
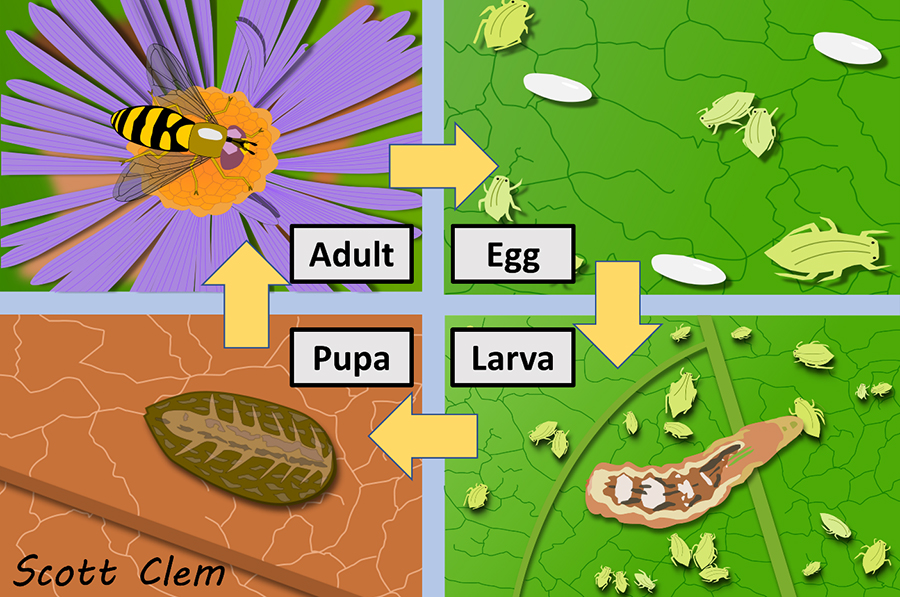
Figure 3 depicts the Eupeodes americanus life cycle and is representative of most flies from the subfamily Syrphinae. Adult flies seek out colonies of herbivorous aphids and will lay an appropriate number of small, white, oval-shaped eggs among them. Tiny first-instar larvae hatch a few days later and immediately begin feeding on the aphids. Larvae become progressively larger as they advance through five larval instar stages. Once they reach the fifth stage they pupate and eventually become an adult to begin the process over again. Most of the time, males and females are easy to distinguish: females have a distinct gap between the eyes dorsally (situated near the back) and males are holoptic, meaning that their eyes are touching. This is also true for many members of the subfamily Eristalinae, as well as other fly families.

Group 1 Species Descriptions


Allograpta
Allograpta species are easily recognized by the four dorsal yellow bands at the tip of the abdomen, two parallel vertical bands, and two diagonal tear-drop shaped bands. Both A. exotica (4.7–8.5 mm) and A. obliqua (5.4–9 mm) commonly occur in Georgia, although A. obliqua is most common. Allograpta exotica tends to be smaller, lighter in color, and has slightly different thoracic and abdominal patterning. Specifically, the vertical bands at the tip of the abdomen are often connected to the middle bands, and the second abdominal tergite is black and lacks a small yellow strip (see arrows in photos).

Sphaerophoria
Sphaerophoria are slender flies with horizontal yellow-black bands on the abdomen. These are most similar to Allograpta species, but they lack the four bands at the tip of the abdomen. They also look similar to, but typically much smaller than, Eupeodes or Syrphus species. Known as globetails, males (see inset image) have a characteristic globular-shaped tip to the abdomen, which is absent in females. There are at least 10 species in eastern North America, but S. contigua (6.5–8.8 mm) is by far the most likely to be spotted in the Southeast. It is relatively uncommon in Georgia and further south.

Eupeodes
The genus Eupeodes is most easily distinguished from similar genera (like Syrphus) by having a black-margined abdomen (see photo). This character is especially apparent when examined from below. Several species occur in the eastern U.S. but Eupeodes americanus (7–11 mm) is the most common in Georgia. The less common very similar species E. pomus (6.8–12 mm) may also be encountered but is only distinguishable based on male genitalia (see Skevington et al. 2019). Eupeodes americanus exhibits long-distance migration and will fly hundreds of miles from northern latitudes to the southeastern U.S. during autumn (Clem et al. 2022a, Clem et al. 2022b). In Georgia, these flies are most commonly found in the fall and spring.

Syrphus
The genus Syrphus is superficially similar to Eupeodes except that it lacks the black-margined abdomen. At least five species occur in Georgia: S. torvus (8.6–13.3 mm), S. vitripennis (7.6–11.4 mm), S. ribesii (8.1–13.3 mm), S. knabi (7.2–12.9 mm), and S. rectus (7.2–10.3 mm). These species can be distinguished by a combination of wing, leg, and antennal characteristics (see Skevington et al., 2019; Vockeroth, 1992). Members of the similar genus Epistrophe also occur in the state, although they are less common. Like E. americanus, some Syrphus species also exhibit long-distance seasonal migration. Examine these specimens carefully as they resemble some eristaline hover flies such as Eristalis transversa and Helophilus fasciatus (see Group 2).

Xanthogramma
Xanthogramma flavipes (7.3–12.3 mm) is not frequently encountered in Georgia but is easily distinguishable by the lateral yellow stripes on the scutum, and by black, yellow, and brownish markings on the abdomen. Larvae of this species are unknown, but they probably feed on underground root aphids tended by ants (Skevington et al., 2019). This species is relatively uncommon in Georgia and further south.




Toxomerus
Toxomerus species are almost certainly the most common hover flies encountered throughout North America. In Georgia and across the East Coast, T. geminatus (6.1–7.6 mm) and T. marginatus (4.9–5.7 mm) are perhaps the most common species encountered year-round, with T. politus (7–9 mm) and T. boscii (4.7–6.8 mm) also occurring regularly. T. geminatus and T. boscii both have distinctive spade-shaped markings on the abdomen but differ in the amount of yellow at the edge of each tergite. T. marginatus is highly variable in coloration but always has a (somewhat subtle) yellow-margined abdomen. T. politus has medial quadrangular markings on the abdomen, often with a bluish hue. This species is unusual because the larvae feed on pollen and sap (especially corn and sorghum).
Other less-common species encountered in Georgia but not pictured here include T. verticalis, T. corbis, and T. jussiaeae. See Skevington et al. (2019) and Vockeroth (1992) for details.

Paragus
Paragus is recognizable as having a short, stout, and punctate (broken into intervals) appearance with a blackish-silver coloration and a black or orange abdomen. It is uncertain how many species occur in Georgia, as numerous species are only identified via male genital dissection (see Skevington et al., 2019; Vockeroth, 1986 and 1992). Paragus haemorrhous (4.3–5.9 mm), however, is perhaps the most common and quite recognizable. This species has a completely black scutellum (hard scale or plate on the thorax of an insect; see arrow in photo) whereas other species have a narrow orange stripe on the tip of the scutellum.

Platycheirus
Platycheirus is a highly diverse genus, with at least 75 species in the Nearctic and 44 in northeastern North America, several of which could occur in the Southeast. Many can be identified by paired rectangular markings on the abdomen that are usually either yellowish or bronzish. Platycheirus quadratus (7.1–9.1 mm; main image) and P. obscurus (6.8–9.4 mm; inset image) are perhaps the most frequently encountered species in Georgia. Species can be identified using a variety of characteristics largely based around the shape of male front legs (see Young et al., 2016; Skevington et al., 2019).



Ocyptamus, Pelecinobaccha, and Pseudodoros—the Slender-Shaped Hover Flies
Numerous “slender” hover flies occur in Georgia. Among them, Ocyptamus fuscipennis (6.8–11.3 mm) is probably the most common and can easily be distinguished by the heavily darkened wing pattern. In contrast, Pelecinobaccha costata (8.7–11.5 mm) exhibits darkened wing pigment only on the anterior edge while Pseudodoros clavatus (7–12 mm; synonym Dioprosopa clavata) exhibits even less of this characteristic. Additional abdominal shape characteristics and patterning differences are also evident.
Four other species, Baccha cognata, Ocyptamus dimidiatus, Hypocritanus fascipennis, and Victoriana attenuata (not pictured here) may also be encountered in Georgia, but less frequently. See Skevington et al. (2019) for additional information.
Subfamily Pipizinae

Pipiza
Pipizinae is a small group that was only recently defined as a subfamily. These flies are similar to Syrphinae in that they have predacious larvae that attack aphids and other soft-bodied insects, but Pipizinae tend to specialize on gall-forming aphids and other arboreal prey. Members of this genus are all somewhat uncommon.
In Georgia, Pipiza femoralis (6.5–9.5 mm) is perhaps the easiest to recognize and possibly the most common. Pipiza species often have large, pale, nearly rectangular markings on the abdomen. Other genera in this group—also rarely encountered in Georgia, including Heringia, Neocnemodon, and Trichopsomyia—do not have these markings and are nondescript compared to many other hover flies. Little is known about most Pipizinae and several may be of potential conservation significance (Clem et al. 2023).
Groups 2–3: Species with Nonpredacious Larvae
Subfamily Eristalinae
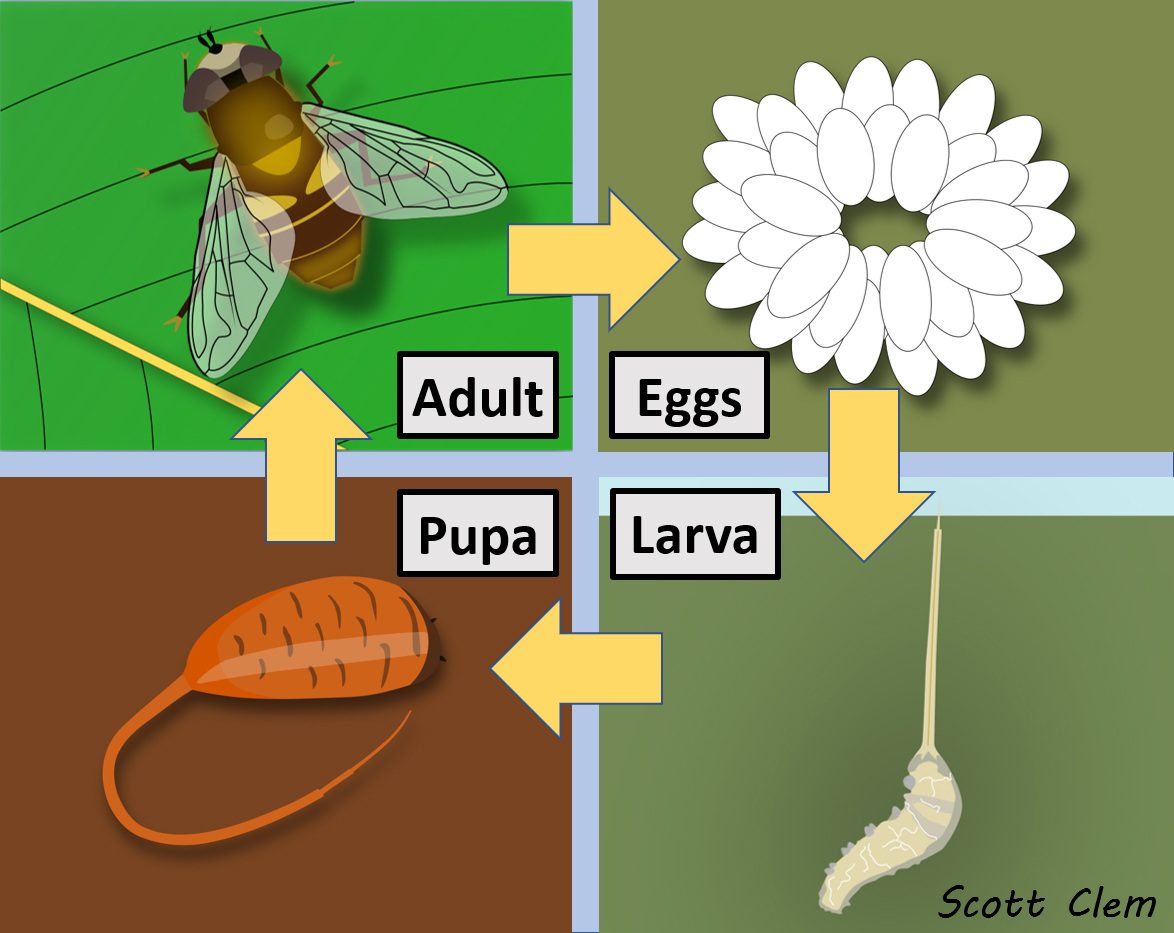
Although life cycles among the Eristalinae are incredibly diverse, Figure 15 is representative for many species. Adults lay eggs in various forms of decomposing organic debris, such as rot holes in trees, decaying wood, sewage dumps, manure piles, compost heaps, etc.
Larvae of many species act as filter feeders, feeding on microorganisms in their environment. Several species also have long breathing siphons (hence the name “rat-tailed maggot”) which helps them breathe in oxygen-deficient (anoxic) conditions. Once they reach the fifth larval stage, they crawl away from their mucky habitats to pupate. Later they will emerge as adults, mate, and begin the process over again.
Like the Syrphinae, males and females often exhibit sexual dimorphism,meaning that males and females look different. Females often have a distinct gap between their eyes and the males are holoptic (eyes touching dorsally), although this is not the case in all Eristalinae. In many cases, such as with Eristalis sp., males tend to have unique abdominal patterns not present in females. Males often establish mate-seeking territories that they aggressively defend against rivals. Rat-tailed maggots are only one of several types of eristaline larvae (Figure 16).

Group 2 Species Descriptions: Wasp-Like Mimics


Copestylum
Copestylum flies have a noticeably short and stout appearance, but perhaps their most noticeable feature is the presence of a distinct facial protrusion (see arrow, Figure 17). Copestylum vesicularium (7.4–10.4 mm) and C. vittatum (8.1–8.7 mm) are the most common species encountered in Georgia. Whereas C. vesicularium is dark and iridescent, C. vittatum is black and yellow striped with two round, yellow spots on the scutum. At least two other species (C. sexmaculatum and C. barei) may be found in Georgia and the Southeast, but they are uncommon (see Skevington et al., 2019, for details). Larvae of some Copestylum develop in plants of the family Bromeliaceae. The western species C. mexicanum (a rare vagrant in Georgia) feeds on decomposing cacti.


Helophilus and Parhelophilus
Helophilus fasciatus (10.8–15.2 mm) and Parhelophilus species bear a resemblance to each other because they have longitudinal stripes on the thorax and yellow markings on the abdomen. The more common Helophilus fasciatus, however, is larger and fairly easy to distinguish with its elongated darkened wing spot known as a pterostigma (see arrow, Figure 18). Parhelophilus is less common but two species can be encountered in Georgia: P. integer (8.7–10.9 mm) and P. flavifacies (9.7–11.7 mm; not pictured). Larvae of both genera are aquatic and associated with decaying vegetation and stagnant or slow-flowing water. Note that other less-common species may also be encountered (see Skevington et al. 2019).


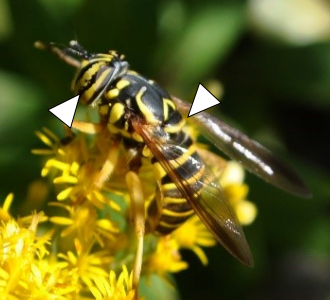


Meromacrus, Milesia, Spilomyia, and Temnostoma—the Extreme Wasp Mimics
Meromacrus, Milesia, Spilomyia, and Temnostoma all contain species that are large, highly convincing wasp or hornet mimics. Meromacrus acutus (13.3–18.6 mm) and Milesia virginiensis (16.5–23.6 mm) are the only members of their respective genera found in Georgia and are among the largest hover fly species in North America. Both exhibit thoracic and abdominal patterning that makes them hard to confuse with any other species. M. virginiensis is a mimic of southern yellow jacket queens and is known for its curious habit of hovering in front of people. It is this behavior that has coined them the name “news bees” or “good news bees.” If one lands on you, it is believed to be good luck.
At least three species of Spilomyia are known to Georgia, including S. longicornis (12.4–16.2 mm), S. texana (not pictured), and S. alcimus (not pictured). These species visit goldenrod (Solidago sp.) and other fall-flowering plants and immediately stand out with their dramatic striped eye patterning. S. longicornis (12.4–16.2 mm) is the most commonly encountered species and it is best distinguished from S. texana and S. alcimus by its yellow-rimmed scutellum (see arrow, Figure 19). Larvae are likely to be found in rot holes of deciduous trees. For distinguishing New World Spilomyia species see Thompson (1997).
Several species of Temnostoma may occur in Georgia, but three species are most common: T. daochus (9.5–14.5 mm), T. balyras (8.5–11.5 mm), and T. trifasciatum (not pictured). These species may look similar to Spilomyia but do not have the striped eye patterning. T. daochus is best distinguished from similar species by having a yellow-margined scutellum (see arrow). T. balyras and T. trifasciatum are both dark with yellow bands on the abdomen and are difficult to distinguish in the field (see Skevington et al. 2019). Larvae of these species may be found in rotting wood.


Syritta and Tropidia
Syritta and Tropidia species are grouped together here because they are both small, black flies with enlarged hind femurs (also known as the metafemur) and similar abdominal patterning/shape. However, Tropidia species are larger and have a large triangular process (a projection or outgrowth of tissue) on the metafemur while Syritta species lack this process and have a metafemur with a unique orange/black color pattern. Both Syritta pipiens (6.5–9.5 mm) and S. flaviventris (6.9–9.4 mm; not pictured) occur in Georgia and they are distinguished by the presence of a basal peg (a short distinct projection) on the metafemur of male S. flaviventris. Both of these are introduced species and can be found on many continents around the world.
Tropidia albistylum (10.9–13.4 mm) is by far the most common Tropidia in Georgia and it can be distinguished from other similar species by the yellow coloring at the base of the metafemur [see arrow; not present in the common northern species Tropidia quadrata (not pictured)] and the large pale patterns on the abdomen [smaller in the rare species Tropidia calcarata and Tropidia mamillata (not pictured)]. Larvae of Syritta and Tropidia may be found in decaying organic matter. Syritta larvae are particularly fond of compost or manure.


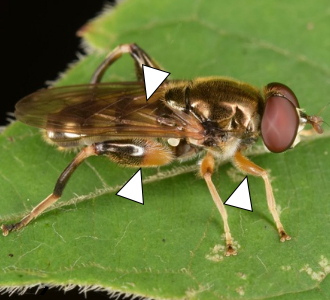

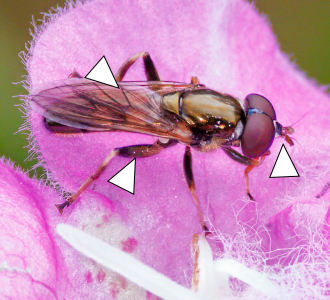
Blera, Chalcosyrphus, and Xylota spp.
Blera, Chalcosyrphus, and Xylota are three similar genera that encompass nearly 40 species in eastern North America. Larvae often live in decaying wood and adults tend to visit flowers in forested landscapes, often near water. Although many species are fairly distinct, several can be difficult to distinguish in the field. They generally exhibit black/silver, gray, and orange/yellow/pale color patterns and occasionally have prominent facial protuberances. The five exemplar species pictured here are encountered in the Southeast: Blera pictipes, Chalcosyrphus metallicus, Chalcosyrphus chalybeus, Xylota bicolor, and Xylota ejuncida. These species demonstrate the range of morphological diversity of these genera.
B. pictipes (7.6–10.3 mm) has a prominent facial protuberance and a distinct yellow mark on the side of the thorax (see arrow, Figure 21). C. chalybeus (8.5–11.5 mm) is almost entirely a steely blue-tinged black. C. metallicus (8.1–10 mm) has four pale spots on the abdomen, solid yellow front legs, and a bicolored hind femur (see arrows, Figure 21). X. bicolor (13.8–14.7 mm) has a nearly solid orange abdomen. Finally, X. ejuncida (9.6–10.9 mm) also has four pale spots on the abdomen (similar to C. metallicus) but lacks a bicolored hind femur and has a shiny frontal triangle on the head (see arrows, Figure 21).
Pollinator surveys in apple, peach, and blueberry orchards have recorded large numbers of this species (McCravy et al., 2024). Note that many other species and genera may be encountered in Georgia. See Skevington et al. (2019) for details.
Group 3 Species Descriptions: Bee-Like Mimics




Mallota, Merodon, and Pterallastes—the Extreme Bumblebee Mimics
Mallota, Merodon, and Pterallastes are grouped together because they are particularly compelling bumblebee mimics. Mallota spp. are perhaps the most compelling. Mallota bautias (10.5–19.5 mm) and M. posticata (12–18.5 mm) are the most commonly encountered species of this genus in Georgia. M. posticata usually has yellow pile (fuzz) on at least the front part of its abdomen, whereas M. bautias has a much blacker abdomen (Figure 22). These species also have enlarged distinct hind femurs. Larvae are filter-feeding rat-tailed maggots often found in tree holes with stagnant water (also known as rot holes). Images: Katja Schulz (M. bautias) Lee Elliott (M. posticata).
Merodon equestris (12.3–17.2 mm) is another bumblebee mimic but this species tends to be sporadically seen in urban or suburban habitats or areas with cultivated plants. Known as the Narcissus Bulb Fly, they are minor ornamental plant pests because larvae (Figure 16) feed on bulbs of daffodil (Narcissus) and relatives. Color morphs range from dark to light and there is much diversity in between. However, they tend to be covered in pile and do not have the same massive hind femur found in Mallota species. Image: Rich Stevenson.
Pterallastes thoracicus (10.7–13.2 mm) is the only member of its genus in North America and is most often encountered in northern Georgia. This species is recognizable with a thorax covered in dense, fine yellow hair, a black abdomen, and a lack of enlarged hind femurs (compared to Mallota). Image: Bryan E. Reynolds.

Orthonevra
Orthonevra nitida (4.5–5.5 mm) is by far the most common member of its genus in the southeastern U.S. This small fly is easily recognized by its striking complex eye patterns, which are not found among other members of its genus in Georgia. Orthonevra typically are associated with wetlands and marsh-style habitats. For information on other less common species see Skevington et al. (2019).

Eristalinus
Eristalinus aeneus (8.4–11.7 mm) is an introduced species and the only member of its genus encountered in most of the United States. While extremely common further north, it is relatively uncommon in Georgia. This species is immediately recognizable by its shiny silvery coloration and spotted eyes. Larvae are found in sewage ponds and lagoons and are important in waste recycling.




Eristalis
The genus Eristalis (drone flies) consists of many large, common hover flies, most of which are found visiting a wide variety of flowers in a wide range of habitats. All species are thought to have rat-tailed maggot larvae (Figure 16), which are decomposers and can be found in aqueous and semi-aqueous environments. At least 12 species can be found in eastern North America, five of which are commonly encountered in Georgia: E. arbustorum, E. dimidiata, E. saxorum, E. tenax, and E. transversa. These species can be found year-round in the Southeast but are most abundant during fall and spring.
Eristalis arbustorum (8.3–12 mm), known as the European Drone Fly, is most likely to be encountered in northern Georgia and is very common in northern states. This species resembles E. dimidiata and E. saxorum but is usually smaller and has a uniformly pilose (hairy) face. Males have a conspicuous hourglass shape on the abdomen (see arrows, Figure 25), which is absent in females. A very similar native species, Eristalis brousii (not pictured) was once very common but has largely been displaced by the introduced E. arbustorum.
Eristalis dimidiata (10.1–14 mm) is most easily recognized by its black sleek abdomen and darkened wing bases (see arrow, Figure 25). Eristalis saxorum (11.3–13.7 mm; not pictured) possesses similar features but has a distinct bluish hue.
Eristalis tenax (11.7–15.8 mm) known as the Common Drone Fly is an introduced species notable for being a particularly convincing honeybee mimic. They are commonly misidentified as such in news articles and media about honeybees. On occasion, they can even be found visiting flowers in large numbers, much like honeybees. The existence of this species has spurred the roughly 2000-year-old “Bugonia Myth” in which people believed in the spontaneous generation of honeybees from animal carcasses (Shipley 1918). This myth is found in a variety of literary works, including poetry, Shakespeare, and the Bible in the form of Samson's riddle (Judges 14:14): “Out of the eater something to eat; out of the strong something sweet.” This species, and likely other Eristalis, are also interesting because they can cause intestinal myiasis (infestation with fly maggots) in humans and animals. In rare instances of accidental ingestion, larvae can wreak havoc on the body.
Eristalis transversa (10.1–12.2 mm) is a native species that is very common and fairly conspicuous. It is a generalist found feeding on many species of prairie flowers, but they seem to have a particular affinity for Black-eyed Susans (Rudbeckia hirta) and similar flowers. The bright yellow coloration, particularly on the scutellum, differentiates this species from other Eristalis.
Eristalis stipator (9.8–14.8 mm; not pictured) is more common in western North America but is a vagrant in the eastern U.S. It can be distinguished from E. dimidiata and E. saxorum by its long white curly pile on the third and fourth abdominal segments. It also lacks darkened wing bases.



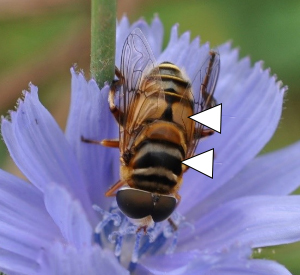
Palpada
Palpada is one of the few genera that has greater diversity in the South compared to the North (this is also true in Toxomerus, Allograpta, and Copestylum). At least seven species of Palpada have been recorded in Georgia, but P. furcata (8.4–10.4 mm) and P. vinetorum (10–13.5 mm) are particularly common, followed by P. pusilla (9.1–12.2 mm), P. agrorum, and P. albifrons (8–10 mm; not pictured). These species are most easily distinguished by patterns and coloration of the scutum and scutellum (see arrows, Figure 26). Palpada furcata has a distinct gray scutum with black longitudinal stripes whereas P. pusilla is distinctly bicolored. Palpada vinetorum and P. agrorum are perhaps the most similar, both with black and whitish scutal bands and large orange-yellow abdominal markings, but P. vinetorum tends to be less dark with a duller shade of orange compared to P. agrorum. Palpada albifrons is a coastal species with a bicolored scutum like P. pusilla but with a more creamy-yellow coloration. Two other species, P. texana and P. furcata, have rarely been spotted in Georgia and are probably western vagrants. Palpada larvae are aquatic filter feeders.
Subfamily Microdontinae

Microdon spp.
Microdontinae is a small subfamily of rare flies that are most well known for their larval habits as ant-nest brood parasites (Figure 28). These flies are rarely encountered and little is known about the geographic distribution and habits of most species. They are in the extreme minority of hover flies that do not visit flowers as adults. This makes it difficult to predict where and when to find them.
Microdon is the most diverse genus in this subfamily and probably the most commonly encountered in North America. They are large, robust, and often pilose (fuzzy) and/or metallic flies distinguished by their elongated antennae and somewhat flattened faces. Microdon megalogaster (12.5–15.7 mm; Figure 27) is among the more common species found in Georgia and may be distinguished by the yellow pilose scutum and black pilose abdomen reminiscent of bumble bees. Laetodon, Mixogaster, Omegasyrphus, Rhopalosyrphus, Serichlamys (not pictured), and other Microdon species may also be found in Georgia. Consider yourself lucky if you encounter any of them! For species identification see Thompson (1981) and Skevington et al. (2019).

Hover Flies Not Pictured but Found in Georgia
Does the specimen you are identifying not match any of the pictures? Here is a list of genera/species that may be encountered in Georgia but are not pictured or discussed in this guide. These are not commonly seen, because either they occupy specialized habitats or niches, or Georgia is at the edge of their range. See Miranda et al. (2013) and Skevington et al. (2019) for details.
Syrphinae
Chrysotoxum plumeum, Dasysyrphus venustus, Didea fuscipes, Doros aequalis, Epistrophe spp., Epistrophella emarginata (perhaps not too uncommon), Megasyrphus laxus, Melangyna fisherii, Melanostoma mellinum, and Meliscaeva cincella.
Eristalinae
Brachyopa spp., Brachyopalpus oarus, Callicera erratica, Ceriana willistoni, Cheilosia spp., Chrysogaster spp., Cynorhinella longinasus, Eumerus funeralis (minor horticulture pest, not too uncommon), Ferdinandea buccata, Hadromyia spp., Hiatomyia cyanescens, Lejota aerea, Myolepta spp., Nuntianus cubanus, Pelecocera pergandei, Psilota spp., Rhingia nasica, Sericomyia spp., Somula decora, Sphecomyia vittate, Sphegina spp. (perhaps not too uncommon), Teuchocnemis spp., and Volucella evecta.
Acknowledgments
Thank you to the multitude of citizen scientists on iNaturalist (https://www.inaturalist.org/observations?place_id=23&taxon_id=49995&view=species) and BugGuide (https://bugguide.net/node/view/196) for providing observational records useful for the development of this manuscript. Also thank you to all the photographers who granted us permission to use their images. Finally thank you to Joe McHugh, Jim Hogue, Jason Schmidt, and Andrew Young for providing helpful reviews of this publication. Funding for this publication was provided by the USDA National Institute of Food and Agriculture Postdoctoral Fellowship Award 2021-67034-35132.
Author C. Scott Clem is now an assistant professor of Ecology at the Illinois State University School of Biological Sciences (contact cscottclem@gmail.com or visit his website: c-scott-clem-entomology.me).
References
Bugg, R. L., Colfer, R. G., Chaney, W. E., Smith, H. A., & Cannon, J. (2008). Flower flies (Syrphidae) and other biological control agents for aphids in vegetable crops (Publication No. 8285). University of California Division of Agriculture and Natural Resources Cooperative Extension. https://anrcatalog.ucanr.edu/pdf/8285.pdf
Clem, C. S., Hart, L. V., & McElrath, T. C. (2023). A century of Illinois hoverflies (Diptera: Syrphidae): Museum and citizen science data reveal recent range expansions, contractions, and species of potential conservation significance. Journal of Insect Science, 23(4). https://doi.org/10.1093/jisesa/iead051
Clem, C. S., Hobson, K. A., & Harmon-Threatt, A. N. (2022a). Insights into natal origins of migratory Nearctic hoverflies (Diptera: Syrphidae): New evidence from stable isotope (δ2H) assignment analyses. Ecography, 2023(2), e06465. https://doi.org/10.1111/ecog.06465
Clem, C. S., Hobson, K. A., & Harmon-Threatt, A. N. (2022b). Do Nearctic hoverflies (Diptera: Syrphidae) engage in long-distance migration? An assessment of evidence and mechanisms. Ecological Monographs, 92(4), e1542. https://doi.org/10.1002/ecm.1542
Duffield, R. M. (1981). Biology of Microdon fuscipennis (Diptera: Syrphidae) with interpretations of reproductive strategies of Microdon species found north of Mexico. Proceedings of the Entomological Society of Washington, 83, 716–724. https://www.biodiversitylibrary.org/page/16365065
Dunn, L., Lequerica, M., Reid, C. R., & Latty, T. (2020). Dual ecosystem services of syrphid flies (Diptera: Syrphidae): Pollinators and biological control agents. Pest Management Science, 76(6), 1973–1979. https://doi.org/10.1002/ps.5807
Jauker, F., & Wolters, V. (2008). Hoverflies are efficient pollinators of oilseed rape. Oecologia, 156, 819–823. https://doi.org/10.1007/s00442-008-1034-x
Marshall, S. A. (2012). Flies: The natural history and diversity of Diptera. Firefly Books. https://www.fireflybooks.com/BookDetails?Pid=28
McCravy, K. W., Clem, C. S., Bailey, J. B., Elgar, S. A., & Blaauw, B. R. (2024). Hover fly (Diptera: Syrphidae) diversity and seasonality in North Georgia apple and peach orchards. Journal of Economic Entomology, 117(4), 1572–1581. https://doi.org/10.1093/jee/toae103
Miranda, G. F. G., Young, A. D., Locke, M. M., Marshall, S. A., Skevington, J. H., & Thompson, F. C. (2013). Key to the genera of Nearctic Syrphidae. Canadian Journal of Arthropod Identification, 23. https://doi.org/10.3752/cjai.2013.23
Orford, K. A., Vaughan, I. P., & Memmott, J. (2015). The forgotten flies: The importance of non-syrphid Diptera as pollinators. Proceedings of the Royal Society B, 282(1805), 20142934. https://doi.org/10.1098/rspb.2014.2934
Rader, R., Bartomeus, I., Garibaldi, L. A., Garratt, M. P. D., Howlett, B. G., Winfree, R., Cunningham, S. A., Mayfield, M. M., Arthur, A. D., Andersson, G. K. S., Bommarco, R., Brittain, C., Carvalheiro, L. G., Chacoff, N. P., Entling, M. H., Foully, B., Freitas, B. M., Gemmil-Herren, B., Ghazoul, J. . . . Woyciechowski, M. (2015). Non-bee insects are important contributors to global crop pollination. Proceedings of the National Academy of Sciences, 113(1), 146–151. https://doi.org/10.1073/pnas.1517092112
Ragsdale, D. W., Landis, D. A., Brodeur, J., Heimpel, G. E., & Desneux, N. (2011). Ecology and management of the soybean aphid in North America. Annual Review of Entomology, 56, 375–399. https://doi.org/10.1146/annurev-ento-120709-144755
Schneider, F. (1969). Bionomics and physiology of aphidophagous Syrphidae. Annual Review of Entomology, 14, 103–124. https://doi.org/10.1146/annurev.en.14.010169.000535
Shipley, A. E. (2012). The “Bugonia” Myth. In H. W. Garrod, A. Platt, & H. Jackson (Eds.), The Journal of Philology (pp. 97–105). Cambridge University Press. https://doi.org/10.1017/CBO9781139523875.006
Skevington, J. H., Locke, M. M., Young, A. D., Moran, K., Crins, W. J., & Marshall, S. A. (2019). Field guide to the flower flies of northeastern North America. Princeton University Press. https://doi.org/10.2307/j.ctv7xbrvz
Thompson, F. C. (1981). Revisionary notes on Nearctic Microdon flies (Diptera: Syrphidae). Proceedings of the Entomological Society of Washington, 83, 725–758. https://repository.si.edu/handle/10088/17480
Thompson, F. C. (1997). Spilomyia flower flies of the New World (Diptera: Syrphidae). Memoirs of the Entomological Society of Washington, 18, 261–272. https://repository.si.edu/bitstream/handle/10088/17084/ent_FCT_73.pdf?isAllowed=y&sequence=1
Vockeroth, J. R. (1986). Revision of the new world species of Paragus Latreille (Diptera: Syrphidae). The Canadian Entomologist, 118(3), 183–198. https://doi.org/10.4039/Ent118183-3
Vockeroth, J. R. (1992). The flower flies of the subfamily Syrphinae of Canada, Alaska, and Greenland: Diptera: Syrphidae. Agriculture Canada. https://publications.gc.ca/pub?id=9.811395&sl=0
Young, A. D., Marshall, S. A., & Skevington, J. H. (2016). Revision of Platycheirus Lepeletier and Serville (Diptera: Syrphidae) in the Nearctic north of Mexico. Zootaxa, 4082(1), 1–317. https://doi.org/10.11646/zootaxa.4082.1.1
Status and Revision History
In Review on May 28, 2024
Published on Aug 20, 2024


























































