The term natural enemy comes from the study of biological control or the control of pests by living organisms that regulate pest populations. A natural enemy could eat the pest (predator), live inside of the pest until development is complete (parasitoid), or cause infection in the pest population (pathogens). Natural enemies of pests are valuable to crop production and can help with pest control. Having a diversity of natural enemies and types of natural enemies in a system helps with regulating multiple pest species.
A challenge for modern agriculture is providing the conditions to balance the demands of production with the promotion of natural enemies to provide biological control services. Knowing and recognizing naturally occurring predators and parasitoids is a first step. We provide a brief overview of common groups and then discuss habitat management concepts for conservation.
Common Native Predatory Natural Enemies
This section provides an overview of common predatory arthropod species (insects and spiders) in blueberry systems—including both the vegetation within blueberry fields and the border areas surrounding those fields—of the Southeast, based on information from Extension publications and our recent research in the primary blueberry-producing counties of Georgia (Whitehouse et al., 2018; Schmidt et al., 2019; Schmidt et al., 2021). For more detailed information, please read the referenced publications. Based on this research, we find that these communities of natural enemies currently are composed of many spiders, some insect predators, and few parasitoids.
Araneae: Spiders

Adult spiders range in size from very small to very large. In addition, immature stages of different species range in size and appearance, making it very difficult to distinguish species at immature stages. Some spiders can produce up to 1000 or more eggs and offspring in one season. The functions of spiders in blueberry systems are not well studied, but some species build webs that capture a variety of mobile pests like flies, caterpillars, thrips, and aphids, and other species forage among the vegetation by ambushing prey or chasing them down. Figures 1 and 2 show both the size distribution of spiders and general forms of spiders to help you characterize them easily in the field. Notice that some of the spiders have appendages at the front, resembling additional legs or boxing gloves; these are male spiders. Spiders with thinner legs and smaller bodies often are web-building types, and larger-bodied spiders or those with thicker legs are more commonly found on the ground.

Clockwise from the top center, the families of common spiders represented are: Salticidae, Lycosidae, Araneidae, Salticidae, Oxyopidae, Theridiidae, Salticidae; center: Araneidae.
Neuroptera: Lacewings
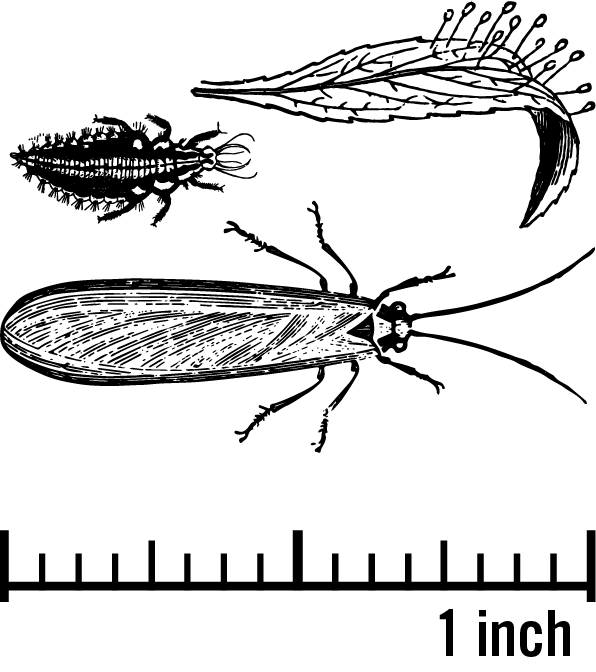
Lacewings are generalist predators of aphids, spider mites, scales, and other small insects. Their size distribution at various life stages is seen in Figure 3. These predators get their name from their thin translucent wings as adults (Figure 4A). The larvae have piercing-sucking mouthparts, and often are referred to as "alligators" (Figure 4B). Figure 4C shows the unique form of their eggs, which look like thin stalks with a ball at the end. These predators live diverse lifestyles. You can find eggs in both crop and noncrop areas. Larvae are voracious predators, while adults feed mostly on nectar and pollen.

Photos (a-c): Melissa Schreiner, Colorado State University, Bugwood.org; Bradley Higbee, Paramount Farming, Bugwood.org; Brett Blaauw.
Hemiptera: True Bug Predators
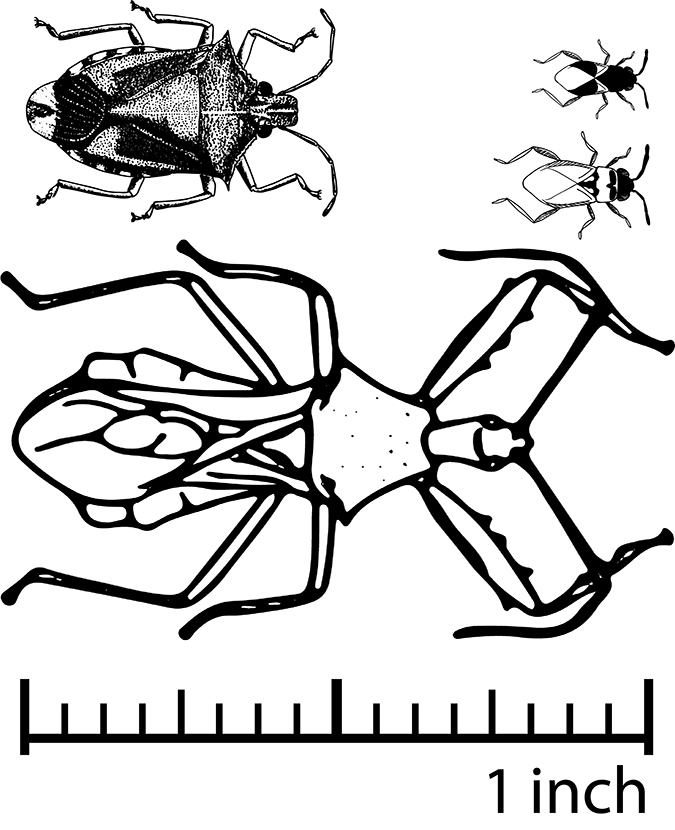
Predators in the Hemiptera order all have piercing-sucking mouthparts. Hemiptera suck the juices out of pest eggs, larvae, and/or adults. Most are generalist predators and feed on a variety of pest species, including Lepidoptera (winged insects) eggs and larvae, aphids, mites, white flies, and plant bugs. Some of the beneficial predaceous Hemiptera can be challenging to discriminate from pests, especially in their immature stages. Figure 5–9 show examples of a range of a few common predaceous Hemiptera, including their sizes and colors.

Photos: Russ Ottens, University of Georgia, Bugwood.org.
Photos (left to right): Russ Ottens, University of Georgia, Bugwood.org; Frank French, Georgia Southern University, Bugwood.org.
Photos: Russ Ottens, University of Georgia, Bugwood.org.

Photos (left to right): Adam Sisson, Iowa State University, Bugwood.org; John Ruberson, Kansas State University, Bugwood.org.
Coleoptera: Ground Beetles and Rove Beetles
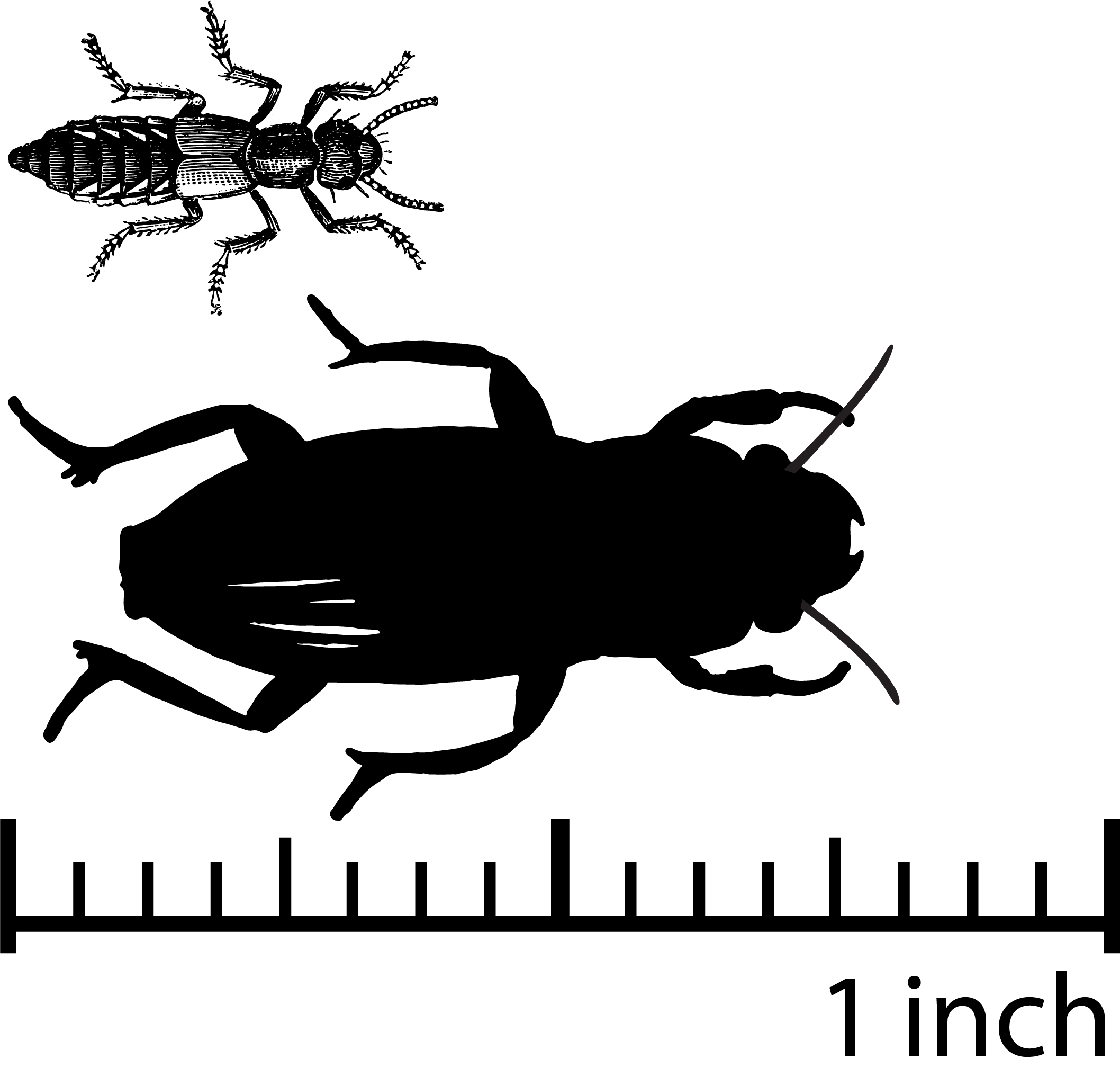
Coleoptera, the order of beetles, is one of the most diverse groups of animals on Earth. These beetles live in many habitats from lakes to crop systems, and on the shores of beaches foraging for insects, decaying material, and seeds. Beetles also are important as nutrient cyclers because they process the dung from vertebrates (Fuzessy et al., 2021). In blueberry systems in Maine, dung beetles potentially reduce disease in blueberries (Peterson, n.d.). Figures 11–12 show a few examples of ground predators and scavenging predators that can be found both on the ground and in the canopy. The rove beetles, or Staphylinidae, occur on the ground but also forage in flowers and vegetation. Some of these predators eat both prey and weed seeds. Our coverage here is limited in scope by what we've observed and reported in recent extension articles. Given the diversity of Coleoptera, there are likely many more to be found.

Photos (left to right): Whitney Cranshaw, Colorado State University, Bugwood.org; Jessica Louque and Smithers Viscient, Bugwood.org.

Photos (left to right): Joseph Berger, Bugwood.org; Merle Shepard, Gerald R.Carner, and P.A.C Ooi, Insects and their Natural Enemies Associated with Vegetables and Soybean in Southeast Asia, Bugwood.org.
Coleoptera: Coccinellidae (family), a.k.a., Lady Beetles
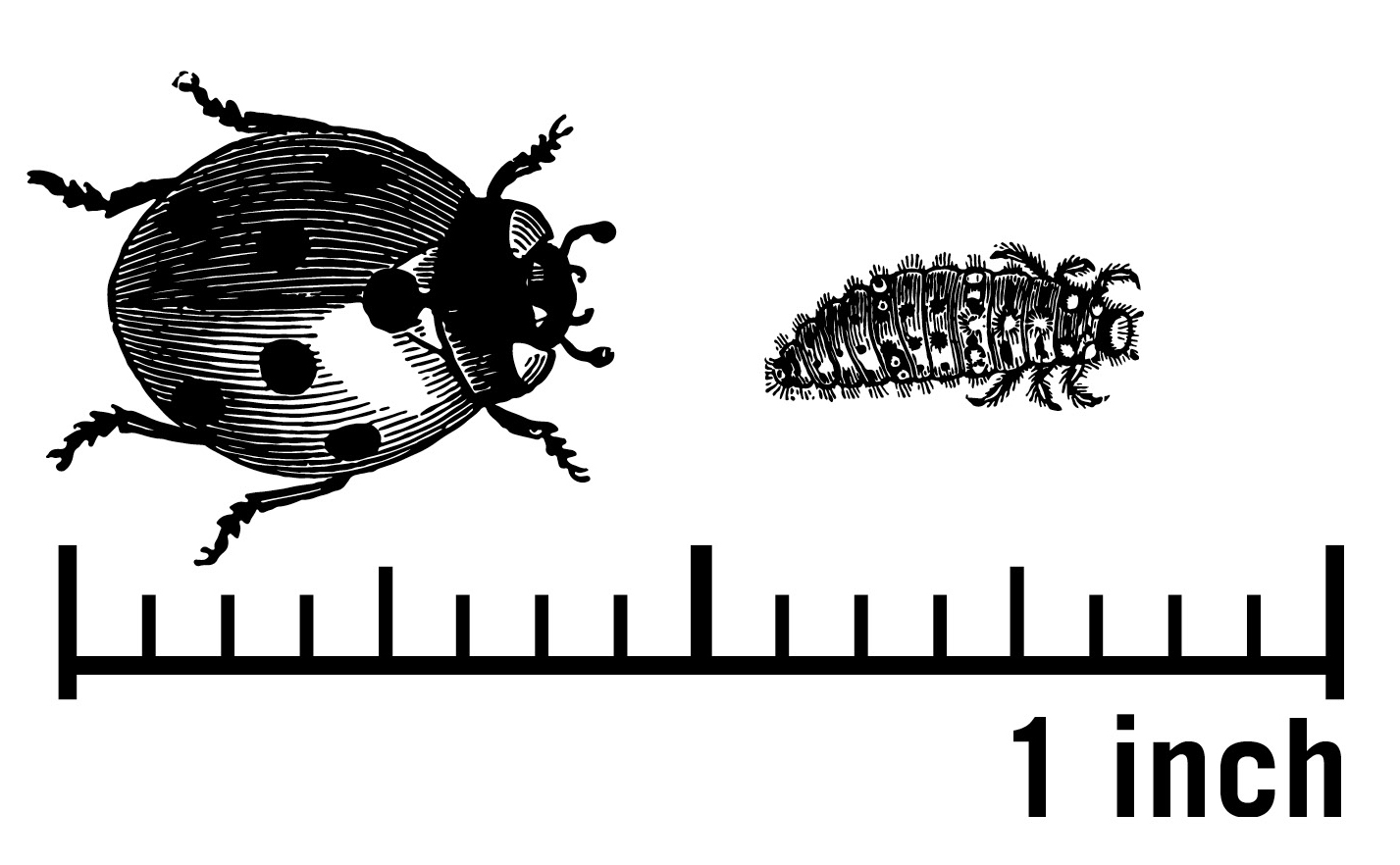
Predators in the family Coccinellidae are the iconic "ladybugs" that we often think of as predators. Ladybug species range in size (about 2 to 10 mm) but are not as variable as hemipteran predators or spiders (Figure 13). The smallest (Scymnus sp.) appear quite different as their larva are covered in white waxy strands (Figure 14).

Photos (left to right): Bodie Pennisi; Jim Jasinski, Ohio State University Extension, Bugwood.org
While known for predation on aphids, ladybugs also eat a range of pest types, including aphids, caterpillar eggs, and spider mites. They also use pollen and nectar as supplemental food sources. Unlike the hemipteran predators, ladybugs develop from eggs to larvae, then pupate into adults (Figure 15). Be on the lookout for mostly yellow- or orange-colored eggs, sometimes as many as 100 eggs on a leaf. Larvae often are black and yellow but vary and some are waxy-looking or spiky-looking (Figure 15B). Adults vary in color and size; learn more about their color diversity, sizes, and ladybug forms in Tomaszewska et al. (2021). The size scale provided in Figure 13 is for larger ladybugs such as the seven-spotted ladybug and the multicolored Asian lady beetle. Other species are smaller.

Photos: A: Bodie Pennisi; B: Susan Ellis, Bugwood.org; C–D: Louis Tedders, USDA Agricultural Research Service, Bugwood.org.
Other Flying Predators: Diptera and Hymenoptera
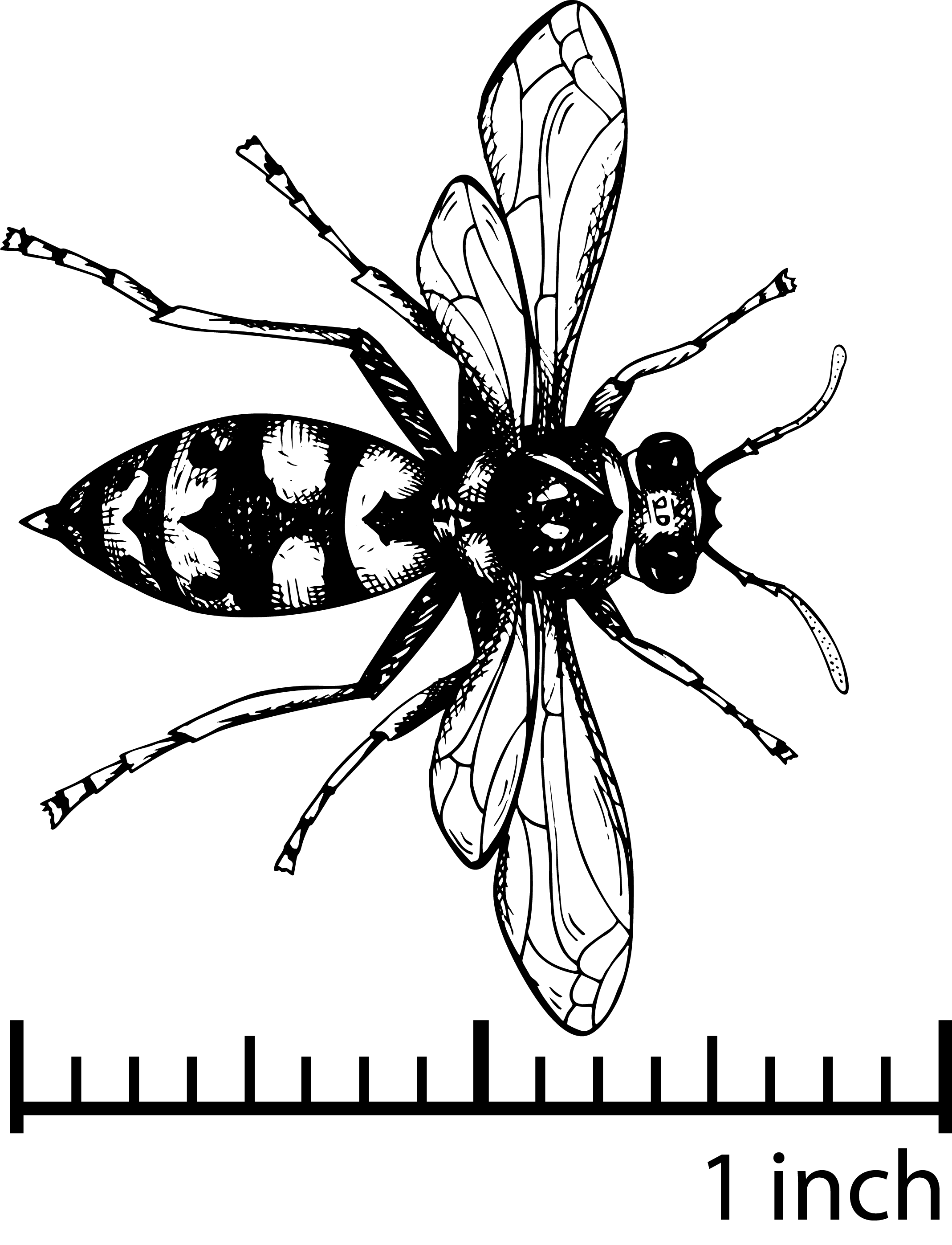
The order Diptera contains a few common natural enemies of pests, like the syrphids, which are also called hoverflies or flower flies (from the family Syrphidae). A diversity of syrphids is found in Georgia, and these flies serve multiple ecosystem functions. As immature larvae (Figure 17)—which look like very small caterpillars—they prey on many small pests on leaves such as aphids, whiteflies, and scales.

Photos (left to right): Eddie McGriff, University of Georgia, Bugwood.org; Bodie Pennisi
As adults (Figure 17), syrphids actively visit flowers and provide pollination services. It is difficult to scout for the larvae, but the adults are easy to recognize in the field by their characteristic hovering flight. They often resemble bees but have only two wings; their eyes are large and meet on the top of the head.

Photos: Bodie Pennisi
Numerous types of predaceous wasps are seen in blueberry systems. Unlike bees, they also eat many types of pests. One common predatory family is Vespidae. Paper wasps, mason wasps, and common yellowjackets belong to this family.
Difficult to See Tiny Predators and EPs
Other Very Small Predators

Photo: ARBICO Organics.
There are many species of tiny predatory mites (< 0.5 mm) that feed on immature stages of insect pests on leaves and in flowers. Predatory mites (Figure 19) move around to find pests and are great natural enemies of other mites. Predatory mites feed on pest mites, thrips, and whiteflies. Studies in Florida have seen success in controlling flower thrips using mites such as Amblyeius cucumeris (Arévalo et al., 2009).
Entomopathogens (EPs)
Researchers are still studying entomopathogenic nemotodes (EPNs) and fungi (EPFs) in blueberry systems. Entomopathogens include fungi, nematodes, viruses, and other organisms that act as pathogens on arthropods such as insects and mites.

Photos (left to right): Peggy Greb, USDA Agricultural Research Service, Bugwood.org; Mark Bailey, University of Georgia, Bugwood.org.
Nematodes and fungi are commercially available. Nematodes are very small (0.3–10 mm, Figure 23) and require moist soil conditions for survival. Nematodes are good at controlling the developing, immature stages of insect pests. EPFs also require moist conditions, and Figure 20B is an example of fungal activity on a Kudzu bug.
Other Native Parasitoids
Hymenoptera Parasitoids
Parasitoids lay eggs on or in a pest species, and then grow by consuming the tissues of the host pest. Because parasitoid communities in blueberry systems of the Southeastern U.S. are quite diverse, we will provide an overview of the groups observed. From our recent studies, we observed more than 20 species occurring within blueberry fields or on the margins of the fields in pine and mixed-wood borders. The more common species, Gonotocerus triguttatus, is a parasitoid of the glassy-winged sharpshooter. Other parasitoids we found mostly belonged to the family Braconidae. All are very small (< 5 mm). The line scale in these figures, if shown, represents 1 mm.

Second photo: Reyes Garcia III, USDA Agricultural Research Service, Bugwood.org. Remaining photos: CNC/BIO Photography Group, Centre for Biodiversity Genomics licensed under CC BY-SA 3.0.
Promoting an abundant diversity of parasitoids will help with biological control of multiple pests. However, parasitoids are sensitive to agricultural practices such as intense management, so efforts should be made to conserve and augment their numbers.
Parasitoids That Effectively Parasitize Spotted-Wing Drosophila (SWD)
Classical biological control programs are currently working to develop solutions for the biological control of an invasive pest, Drosophila suzukii, or spotted-wing drosophila (SWD). Classical biological control is the process of finding natural enemies in the home range of an exotic pest and then performing rigorous tests to ensure those species will not disrupt targeted release areas.
Current work in Georgia and across the world is searching for both resident parasitoids and exotic parasitoids from the native range of SWD. Two species are showing potential for specialized parasitism on SWD and are native to Georgia. In addition, three exotic parasitoids, those from the native range of SWD, are still being researched for their potential in biocontrol programs. All five species are very small (around 1.2–2.5 mm). For further information, see Abram et al. (2022) in the references section.

Photos: CNC/BIO Photography Group, Centre for Biodiversity Genomics licensed under CC BY-SA 3.0.
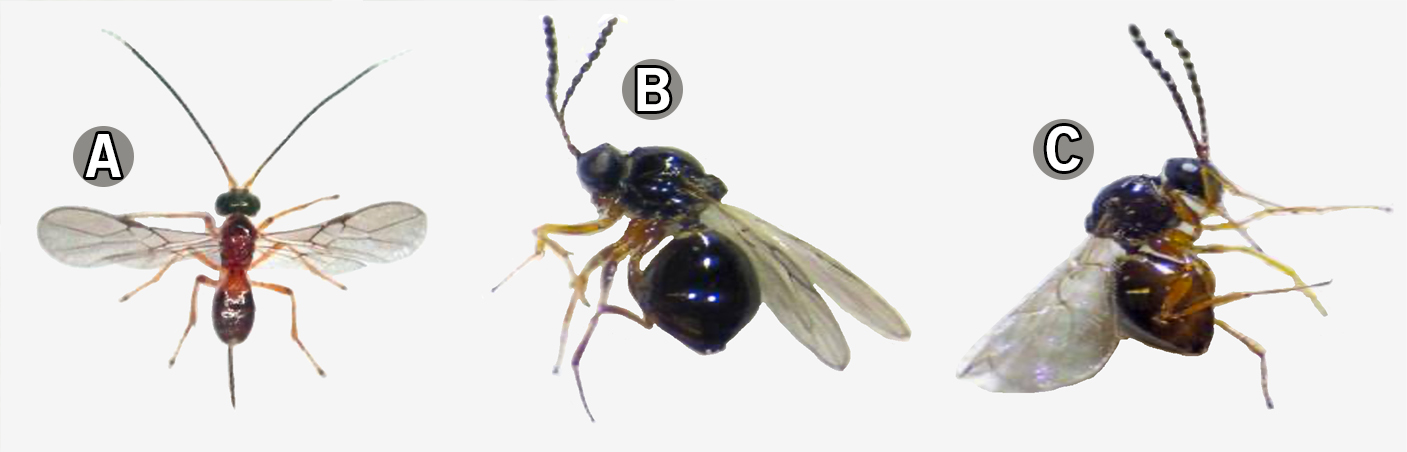
Photos: CNC/BIO Photography Group, Centre for Biodiversity Genomics licensed under CC BY-SA 3.0.
Conservation Concepts for Building Diverse Communities of Natural Enemies
Farmland is nestled among wetlands and river tributaries (Figure 24). Therefore, maintaining biodiversity in Georgia's agricultural landscapes can achieve multiple goals for the conservation of wetlands and waterways throughout the state.
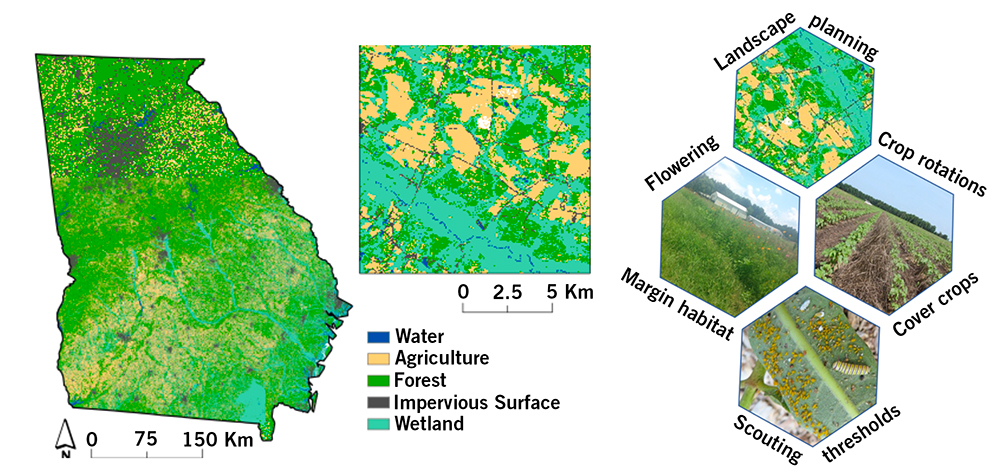
There are many factors to consider in the farm system or farmscape to allow for conservation practices that promote and preserve biodiversity. A multifaceted approach is needed with landscape planning and consideration of profitable space. One conservation strategy is reconsidering marginal land areas of the farmscape or those in which it’s difficult to achieve high yields of a given crop. Such areas could be used for other purposes instead of overinvesting or attempting production when they are likely to produce low yields.
In addition, cover crops may be integrated into the cropping system to provide flowering sources and habitats for pollinators and natural enemies (Mallinger et al., 2019; Bowers et al., 2021). For conservation to work, it’s essential to scout for pests and only spray pesticides when needed (i.e., when pest levels are above economic injury levels).
We can’t cover all the steps for conservation planning in this publication, but UGA Extension and the USDA Natural Resources Conservation Service (NCRS; https://www.nrcs.usda.gov/) can help producers develop plans.
We can provide some concepts to guide placement and elements of habitat. Planning for diversity in the farmscape is an important first step. For example, NRCS recommends at least a 35 ft habitat buffer between a stream or watered area and cropland (Figure 25). Planting buffer areas with flowering herbaceous plants and grasses, shrubs, and trees is a proven method to ensure that there are flowers to attract bees for pollination and to help support natural enemy populations for improved pest control. In addition, creating buffers helps stabilize the bank of a waterway to prevent erosion and preserve water quality. A similar strategy can be employed to separate land areas to create smaller fields with noncrop habitats containing shrubs, trees, and herbaceous flowering plants.
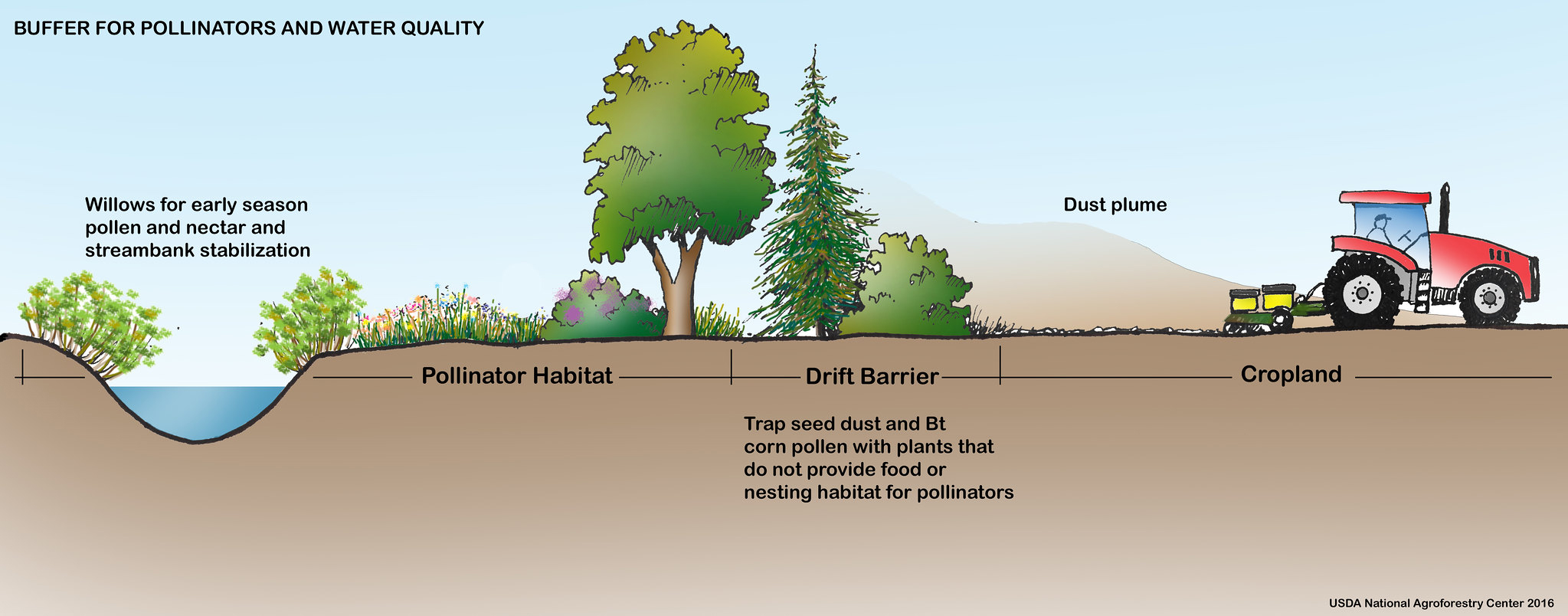
Illustration: U.S. Department of Agriculture National Agroforestry Center, https://www.fs.usda.gov/nac/practices/riparian-forest-buffers.php. More information is available in multiple languages [https://www.fs.usda.gov/nac/buffers/index.html].
Plan for diversity within your fields, separation between productive areas, or integration of flowering habitats within the production system to encourage pollinators and natural enemy population growth (Figure 30). Flowering cover crops and cover-crop residue provide habitats and food to support and grow populations of both natural enemies and pollinators.

Flowering strips planted around the borders of fields, between fields or even between rows, in drive rows, and crop separation areas are all viable options for any farm. Even if equipment is driving in some of the areas, there will be areas where equipment does not commonly drive. Noncrop flowering plants can grow in these areas to connect bordering habitats, which provides corridors for pollinators and natural enemies to move into the crop system.
References
Abram, P. K., Wang, X., Hueppelsheuser, T., Franklin, M. T., Daane, K. M., Lee, J. C., Lue, C., Girod, P., Carrillo, J., Wong, W. H. L., Kula, R. R., Gates, M. W., Hogg, B. N., Moffat, C. E., Hoelmer, K. A., Sial, A., & Buffington, M. L. (2022). A coordinated sampling and identification methodology for larval parasitoids of spotted-wing drosophila. Journal of Economic Entomology, 115(4), 922–942. https://doi.org/10.1093/jee/toab237
Arévalo, H. A., Fraulo, A. B., & Liburd, O. E. (2009). Management of flower thrips in blueberries in Florida. Florida Entomologist, 92(1), 14–17. https://doi.org/10.1653/024.092.0103
Bowers, C., Schmidt, J., Thompson, M., Roberts, P., & Toews, M. (2020). Visual reference guide to common predators and pests in Georgia cotton (Publication No. C 1161). University of Georgia Cooperative Extension. https://extension.uga.edu/publications/detail.html?number=C1161
Bowers, C., Toews, M., & Schmidt, J. (2021). Winter cover crops shape early-season predator communities and trophic interactions. Ecosphere, 12(7), e03635. https://doi.org/10.1002/ecs2.3635
Fuzessy, L., Benítez-López, A., Slade, E., Bufalo, F., Magro-de-Souza, G. C., Pereira, L., & Culot, L. (2021). Identifying the anthropogenic drivers of declines in tropical dung beetle communities and functions. Biological Conservation, 256, 109063. https://doi.org/10.1016/j.biocon.2021.109063
Mallinger, R., Franco, J., Prischmann-Voldseth, D., & Prasifka, J. (2019). Annual cover crops for managed and wild bees: Optimal plant mixtures depend on pollinator enhancement goals. Agriculture, Ecosystems & Environment, 273, 107–116. https://doi.org/10.1016/j.agee.2018.12.006
Peterson, H. (n.d). Berries & biocontrol: Current status of biological control prospects of important berry pests. Maine Department of Agriculture, Conservation and Forestry. https://www.maine.gov/dacf/php/pesticides/documents2/presentations/Berries%20and%20Biocontrol%20Presentation.pdf
Ratnasingham, S., & Hebert, P. D. N. (2007). Bold: The barcode of life data system. Molecular Ecology Notes 7(3), 355–364. https://doi.org/10.1111/j.1471-8286.2007.01678.x
Schilder, A., Isaacs, R., Hanson, E., Cline B., & Longstroth M. (2015). A pocket guide to IPM scouting in highbush blueberries (Publication No. E2928). Michigan State University Extension. https://www.canr.msu.edu/resources/a_pocket_guide_to_ipm_scouting_in_highbush_blueberries_e2928
Schmidt, J., Whitehouse, T. S., Green, K., Krehenwinkel, H., Schmidt-Jeffris, R., & Sial, A. (2019). Local and landscape-scale heterogeneity shape spotted wing drosophila (Drosophila suzukii) activity and natural enemy abundance: Implications for trophic interactions. Agriculture, Ecosystems & Environment, 272, 86–94.
Status and Revision History
In Review on May 28, 2024
Published on Sep 13, 2024


























































