Introduction
The peach production season in Georgia spans from early May to mid-August, with 7,700 acres of orchards producing approximately 3.3 tons/acre of fruit (National Agricultural Statistics Service, 2024). Appropriate preharvest and postharvest handling techniques are crucial for maintaining high fruit quality. Factors such as proper temperature management, relative humidity, handling from harvest all the way to the packing house, and the use of suitable packaging are very important to ensure high fruit quality at a store display.
Truly tree-ripe peaches have the highest possible eating quality, but harvesting fully ripe fruit is not practical because of shipping requirements. Thus, the peach industry has to find a balance between the optimal quality and the durability required to harvest, transport, grade, package, and market fruit (Taylor & Rushing, 2012).
Harvesting peaches is not as simple as it might seem since there are many maturity indices, making the harvesting procedure a challenge for harvesting crews. Peaches (melting-flesh cultivars, such as ‘O’Henry’, ‘Georgia Belle’, and ‘Redhaven’) exhibit climacteric ripening, which means that they can continue ripening after harvest , as long as they are picked at a physiologically mature stage. However, there are certain peach cultivars (nonmelting flesh, such as ‘Prince’ and ‘Tropic Beauty’) that do not ripen significantly after harvest.
In all cases, peaches should be harvested early enough to minimize bruising and premature softening during handling and storage. It is important to mention that the maturity index varies depending on the cultivar as well as the destination of the product. Thus, peaches destined for distant markets often are harvested earlier to survive the long trip. Proper training of the harvesting crew is crucial to avoid picking too early—which could result in a lack of sweetness, juiciness, and taste—or picking too late—which will mean softer fruit with a short shelf life. Since not all peaches mature at the same time or in the same way (melting vs. nonmelting flesh), multiple pickings usually are required for each cultivar. Pickers must be selective, removing only fruit that meets the specific maturity indices of each cultivar.
Melters vs Nonmelters
Melting-flesh peaches soften rapidly to a smooth buttery texture and are most desirable for the fresh market. Melting peaches can be freestone or clingstone, which describes whether flesh fibers detach from (freestone) or remain attached to (clingstone) the stone (seed, pit) when fruit is ripe. If melting types are picked and stored incorrectly, their flesh tends to become mealy, dry, and grainy (Figure 1).
Nonmelting-flesh peaches soften gradually to a rubbery texture and are the main peach type used for canning. Nonmelting types are always clingstone. Two other types show less softening potential: clingstone nonsoftening flesh and stony-hard peaches, which are both suitable for fresh eating because of their crisp flesh. The firmer types do not become mealy when left to ripen (Iezzoni, 2019).

Maturity indices are important indicators of the ripeness and quality for all horticultural commodities. For peaches, these indices are used to determine the optimal harvest time, since early or late harvests can result in reduced quality and shelf life. Monitoring these indices can help growers determine the ideal harvest time, which is crucial for achieving the best quality and flavor. Harvesting too early can lead to underripe, tasteless fruit that often lack nutritional value. On the other hand, harvesting too late can result in overripe fruit that are soft, mushy, and prone to spoilage. In addition, maturity indices can help fruit handlers and retailers determine the appropriate storage conditions and shelf life of the fruit, and predict the potential for postharvest disorders such as chilling injury or decay. Overall, monitoring maturity indices in fruit is essential for ensuring the best quality and value for consumers, as well as minimizing waste and losses in the supply chain.
Maturity Sampling
Maturity sampling should start approximately 4 weeks before the estimated time of harvest and continue until harvest. Early on, maturity sampling should be performed weekly, and when fruit is close to its anticipated maturity, every other day (Cai & Farcuh, 2021).
Sampling starts by choosing 10 to 20 representative trees in terms of the crop load and vigor per block per cultivar and rootstock. Avoid choosing trees from the borders of any blocks, as they are likely encountering more traffic, wind, and irradiation, rendering them not representative. Label the sampled tree and keep sampling from the same trees, by the same person if possible (Cai & Farcuh, 2021).
Skin/surface color is the first visual impression that a harvesting crew has in the orchard. It has been found to be the most reliable nondestructive maturity index and the one most easily understood by pickers (Kao et al., 2012). However, it can be a tricky indicator for maturity because of the large surface-color variability. The ground color of peaches (the area of the fruit not showing the typical red blush from sunlight exposure) changes from green to yellow and eventually to orange. A peach that is approaching maturity has a light green ground color. A break in color toward yellow is the first indication of maturity. When it comes to red-skin cultivars, the degree of red coloration is not a good indicator of maturity, so other indicators should be used. Nevertheless, the red skin coloration is an important quality aspect for consumers (Cai & Farcuh, 2021). Factors such as exposure to sunlight, location of the fruit in the tree, nutrient deficiencies, and cultivar/rootstock variation in red skin color might affect the harvesting crew’s perceptions.
Background/flesh color is the color of the fruit interior, under the peel. It is an important maturity indicator, but when used as a harvesting index, keep in mind the destination of the peach that is being harvested. For certain varieties, the moment the flesh color turns from greenish to yellowish (yellow flesh varieties) or into creamy (white flesh varieties) is an indication that the peach is at the ideal condition to be harvested for shipping (Scalisi et al., 2020). Newer varieties with red flesh make this technique difficult to use as a maturity indicator. Because of this, it is important to use a combination of ripening indicators to determine the developmental stage of the fruit.
Flesh Firmness
Flesh firmness is a maturity index that involves the force required to puncture the fruit flesh with a special instrument called a penetrometer. Firmness measurements can be performed easily by handheld or tabletop penetrometers. It is a destructive method and is one of the most important maturity indicators. For melting-flesh varieties, when fruit matures over time, flesh firmness decreases. Nonmelting-flesh types do not soften significantly after harvest. For melting-flesh peaches, the firmer the fruit, the less ripe it is.
Flesh firmness evaluation is a fairly easy procedure which can be performed in the field using low-cost, handheld penetrometers. A very thin part of the outer skin (approx. 1 mm) has to be removed first (Figure 2) and then, an 8-mm diameter plunger should be pushed into the peach flesh to the specific depth marker (Figure 3). Usually, there is an indication at the 12 mm mark on each plunger to avoid sampling errors.
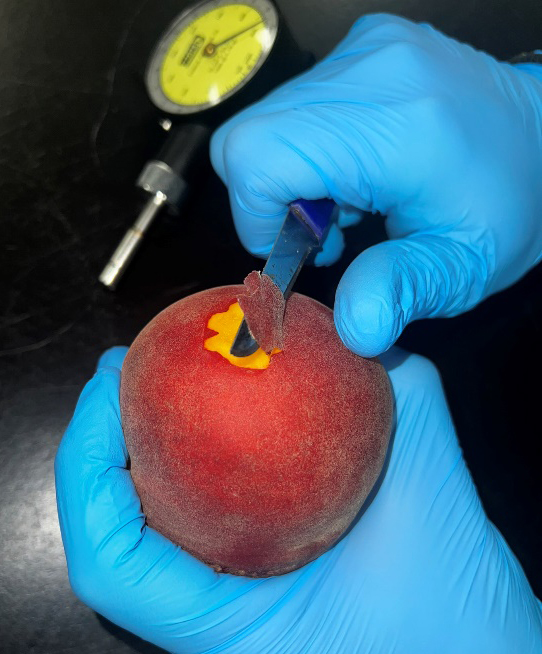

It is important to mention that the person measuring fruit firmness with these handheld penetrometers should place the fruit against a solid, immovable surface (like a tree trunk) to reduce measuring errors. To avoid sampling errors, measurements should be performed by the same person. A representative sample of peaches should be used from multiple trees/locations in order to have a clear picture of the ripeness level of the orchard to be harvested.
Peaches picked for long-distance shipping can be harvested in the 12 to 16-lb firmness range; peaches destined for medium-distance shipping can be harvested in the 8 to 12-lb range and peaches for local sale can be harvested in the 6 to 8-lb range (Table 1). Peaches picked in lower firmness levels will require more care to avoid bruising. When peach firmness falls below 3 lb, extra care is required. In general, fruit with 2–3 lb of flesh firmness are considered ready to eat, while fruit harvested at a firmness below 6–8 lb measured on the cheek have high consumer acceptance (Mitcham, 2023).
| Firmness range (lb) | Ideal shipping range |
|---|---|
| Long-distance chain store | |
| 16–12 | Picking, packing, and transport to regional warehouse |
| 12–8 | (Soft enough for) transport to retail stores |
| 8–6 | Transfer to retail store and put out on display |
| 3–2 | Purchase by end consumer |
| Middle-distance chain store | |
| 12–8 | Picking, packing |
| 8–6 | Transfer to retail store and put out on display |
| 3–2 | Purchase by end consumer |
| Local farm market | |
| 8–6 | Picking and display |
| 3–2 | Purchase by end consumer |
|
This summary is based in part on work and summaries developed by Carlos Crisosto, Kearney Agricultural Center of the University of California. From “Monitoring peach and nectarine ripening,” by W. Shane, 2011, MSU Extension (https://www.canr.msu.edu/news/monitoring_peach_and_nectarine_ripening). |
|
Titratable Acidity and Soluble Solid Content
Titratable acidity is a major component of peach quality which directly affects consumer preferences. It is measured in a laboratory setting using a process called titration. The process utilizes a sodium hydroxide solution of known concentration to a specific pH endpoint, indicated either by the color change of an indicator dye or by a pH meter. Peach acidity decreases over time as the fruit matures and can be measured as titratable acidity (TA) on a percentage scale, correlating with sourness levels. Malic, quinic, and citric are the most abundant acids in peaches (Wu et al., 2022). According to Wang (2009), malic acid has a stronger and more persistent taste compared to citric acid. The use of TA or organic acid levels is not recommended by itself as a maturity index because of the large variation in TA among peach cultivars, which can range from 0.2% to over 1% (Crisosto & Crisosto, 2005). A study conducted by Belisle et al. (2018) in the southern U.S. showed that differences in TA are mainly attributed to the cultivar, not to seasonal variation. Different peach cultivars can be classified into three groups depending on their acidity levels: low, standard/medium, and high (Table 2).
| Low Acidity | Standard/Medium Acidity | High Acidity |
|---|---|---|
| TA < 0.3% or > 4 pH | TA 0.7% or 4 pH | TA > 0.7% or < 3 pH |
| Low SSC | Medium SSC | High SSC |
| 9%–12% | 12%–15% | > 15% |
Soluble solid content (SSC) is another major component of peach quality that affects consumer preference and is linked with sweetness. This quality trait is expressed as a percentage of the juice extract. It is a rough, but accurate, estimation of the sugar levels present in the fruit (among other soluble compounds, such as organic acids, vitamins, minerals, and other dissolved solids). Elevated SSC is associated with higher sugar levels, while low SSC levels have been correlated with a lack of flavor (Cirilli et al., 2016). In peaches, the perception of taste mostly depends on the balance of sugars and organic acids, resulting in sweet and/or sour sensations. It is important to mention that high levels of sugars do not necessarily result in better flavor or higher consumer acceptability.

Measuring SSC is an easy procedure using either an analog (Figure 4) or digital refractometer (Figure 5). Refractometers are simple instruments used for measuring concentrations of aqueous solutions such as gases, liquids, and translucent solids. The extraction procedure for SSC determination is easy and not complicated. To extract juice from the peach, parts of the whole fruit (including peel) can be squeezed using a manual fruit squeezer and strained through a cheesecloth. For higher accuracy, homogenization by grinding the peach pieces in a food processor is recommended. After homogenization, the sample has to be centrifuged for the separation of solid segments from the liquid part (supernatant) and then filtered using a cheesecloth. About two to three drops of the filtered sample should be used (enough to cover the prism) for SSC measurement with both analog and digital refractometers. Peach cultivars can be classified into three broad groups based on their SSC levels: low, medium, and high (Table 2).

SSC to TA ratio is a quality index of high importance, as human taste perception is a combination of sweetness and sourness. The ratio of sugars to acidity increases over time as peaches continue to mature. There is no specific target value for sugars to acidity because of the high variability among varieties. In a study that was published by Huang et al. (2021), it was discovered that the SSC/TA ratio in particular can be significantly impacted by the mineral nutrient content of fruit. Consumer acceptance data has shown that a higher ratio is linked to increased perception of sweetness (Cirilli et al., 2016).
Nondestructive Maturity Indices
Nondestructive maturity indices can allow for large-scale data acquisition or field use to assess the influence of preharvest parameters on fruit maturity and internal quality. Near-infrared spectroscopy (NIRS) is a promising nondestructive option to determine peach fruit quality. It measures the interaction of near-infrared light with the fruit's surface to analyze its composition. By assessing the absorbance and reflectance of light, NIRS can provide information about fruits’ sugar content, acidity, firmness, and other quality parameters related to maturity. There are commercially available handheld devices that use radiation to penetrate the fruit, with their readings changing according to the scattering and absorption of wavelengths. These pieces of equipment require user input and calibration depending on the fruit species/cultivar. Additionally, this technology can be combined with modern sorting lines for large-scale data collection to improve both efficiency and real-time decision-making (Minas et al., 2023).
The delta absorbance (DA) meter is a handheld device that measures the difference of absorbance between 670 and 720 nm. The index of absorbance difference (IAD) correlates the level of actual flesh chlorophyll with fruit ethylene production (Spadoni et al., 2016). This correlation is variety-specific and is linked to consumer acceptability. Moreover, the IAD has a stronger correlation with fruit ripeness than the physicochemical parameters that frequently are used to describe the maturation process.
The preharvest use of the DA meter was initially reported by Ziosi et al. (2008), who verified the index’s capability of detecting physiological changes both early (transition from preclimacteric to the onset of climacteric stage) and late (transition from onset of climacteric to the climacteric stage) during on-tree peach fruit ripening.
The postharvest use of the DA meter has been documented for nondestructive evaluation or prediction of internal quality attributes of peaches at harvest and during storage (Spadoni et al., 2016, and Zhang et al., 2017). Hyperspectral imaging is another nondestructive maturity index that combines imaging technology with spectroscopy to capture detailed spectral information from the fruit surface. This technique allows for the analysis of multiple quality parameters simultaneously, including color, texture, sugar content, and firmness, which are indicative of fruit maturity. Lastly, ultrasound or acoustic resonance testing techniques involve transmitting sound waves through the fruit and measuring the response to evaluate internal fruit properties. By assessing factors like firmness and fruit density, these techniques can provide useful information about fruit ripeness.
Handling fresh market peaches is another key aspect of ensuring a high-quality product since mechanical damage on peaches’ thin skin often diminishes their postharvest quality. Injury from rough harvesting and handling advances the fruit’s senescent phase, causing quality decline and short shelf life. Appropriate handling practices should be followed throughout the postharvest life of the fruit as handling damage acts cumulatively. The physiology of wounded fruit is altered dramatically:
- Respiration increases with a corresponding decrease in shelf life.
- Ethylene (a plant ripening hormone) production increases, accelerating the deterioration of the wounded fruit and adjacent fruit.
- Undesirable color changes occur, such as flesh browning.
- Open wounds are a point of entry for pathogens, leading to decay.
Cooling peaches immediately (or within a few hours) after harvest is important to quickly remove field heat and bring down the overall fruit temperature, ensuring longer shelf life. High temperatures after harvest can damage fruit and—most importantly—hasten the ripening process, further softening the fruit and making it more susceptible to mechanical damage.

With peaches, simply placing warm fruit in a cold storage room is not a very effective way of reducing temperature after harvest. A special method of field-heat removal, often known as precooling, should be employed. The most common precooling methods in the United States are hydrocooling (use of chilled water, used mainly on the East Coast; Figure 6) and forced-air cooling (use of cold forced air through the product, used mainly on the West Coast; Figures 7 and 8). Hydrocooling works more rapidly than forced-air cooling because water has a higher (up to 20 times greater) heat-transfer coefficient than air (Figure 6; Taylor & Rushing, 2012). It is important to continuously maintain the cooling medium close to the target temperature for the fruit, especially during the last portion of the cooling period. The use of modern cooling and storage methods after harvest is key to ensuring higher product quality that can capture and retain a larger market share. Georgia farmers put a lot of effort and resources into harvesting their peaches and need to produce a high-quality product that can compete in the market when compared to peaches from other regions.


Hydrocooling is a quick and efficient precooling method where warm peaches are cooled with the use of chilled, sanitized water. Harvest bins are placed on a conveyor which advances through a shower at 32–33 °F (0–1 °C) ensuring water contact for 30 min. This method gives the producers the advantage of using a sanitizer such as sodium hypochlorite (NaOCl) or peroxyacetic acid (also known as peracetic acid or PAA) to eliminate the possibility of cross-contamination. A major advantage of hydrocooling is that it can rapidly reduce field heat while also cleaning and sanitizing the fruit. However, improper water sanitation has the potential to spread postharvest diseases as well as human pathogens. Despite its efficiency, hydrocooling requires large amounts of water. High operational costs and environmental and water-scarcity issues in many areas make this cooling method not a feasible option for some parts of the country.
Forced-air cooling is a precooling method that utilizes the cold air from the cold room (32–33 °F), creating an airflow through bins or boxes to quickly remove field heat from harvested fruit (Jobling, 2000). Instead of simply setting peaches in a cold room and allowing them to cool down slowly, a tunnel is created by setting two stacks of pallets on a wall that has a special opening (plenum) with a suction fan. A tarp is used to seal the tunnel and create a pressure differential. The pallet layout should ensure that the cold air moves through the openings and around the fruit, rapidly removing the field heat. The warm air is subsequently cooled down by the condenser and released back into the cold room. A disadvantage of forced-air cooling is the requirement of a larger refrigeration system and more floor space to compensate for the increased cooling time (compared to hydrocooling). Additionally, fruit exposed to long sessions of forced-air cooling can become dehydrated. The greatest advantage of forced-air cooling is its low propensity to spread fruit rots (Taylor & Rushing, 2012).
Storage conditions after harvest are very important for the final quality of peach fruit. Peaches have a relatively short shelf life of 2–4 weeks compared to other fruit crops. Fruit that has been stored for too long are unlikely to be tasty or appealing to the consumer. Since peaches destined for fresh consumption display climacteric ripening, they will continue to ripen when picked at the fully mature physiological stage.
Temperature management is the most important factor to consider when it comes to appropriate storage conditions. Avoid the “killing zone” (Figure 9) when cold-storing peaches long-term; this includes a range of temperatures between 36 and 46 °F (2 to 7.7 °C; Warnert, 2005).
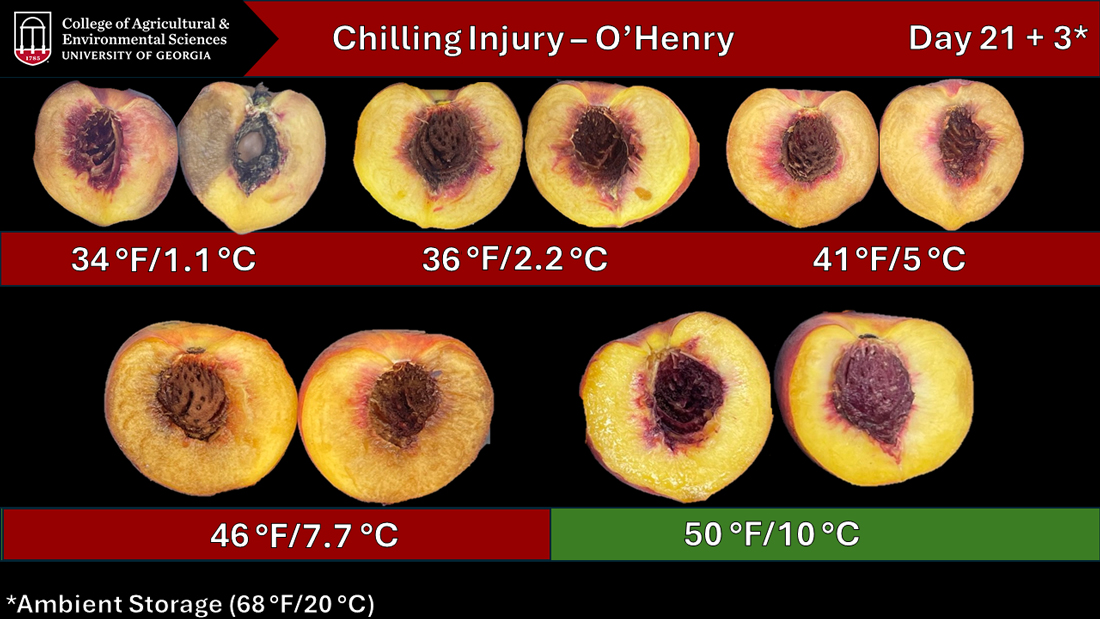
Chilling injury occurs when peaches are kept in low, nonfreezing temperatures between 36 and 46 °F (2 to 7.7 °C). The fruit can develop chilling injury along with other physiological disorders, depending on the varietal tolerance. The failure to soften, darkened pits, reddening or browning of the flesh, and the emergence of a woolly texture will eventually turn the fruit mealy and mushy inside. These symptoms are triggered by the exposure of peaches to temperatures that disrupt normal cell functions, which subsequently reduce fruit quality and marketability (Crisosto & Mitchell, 2002).
Internal breakdown is a disorder that manifests as a brown coloration of the peach flesh close to the pit. Without any obvious symptoms, it may only become visible after the fruit has been cut open. The disorder may have a number of causes, such as calcium deficiencies, fruit maturity, and storage conditions associated with chilling injury (Crisosto et al., 1999). Mealiness or woolliness is a textural defect of peaches that is characterized by dry, crumbly, and nonjuicy flesh. Mealiness or wooliness affects fruits’ eating quality and frequently results in customer dissatisfaction, making this disorder of significant concern to peach growers and distributors (Lurie & Crisosto, 2005).
To avoid these chilling injuries and the subsequent quality disorders, fresh peaches should be cooled promptly and stored at 31–32 °F (-0.6 to 0 °C) avoiding temperature fluctuations over time (Table 3). Fruit that shows symptoms of chilling injury will NOT ripen properly when the storage temperature is increased, resulting in a dry flesh appearance (mealiness) and flesh discoloration (Figure 9). Another factor to consider is the freezing point of peach flesh, which varies between 27 to 30 °F (-2.7 to -1.1 °C), depending on the cultivar and the sugar concentration of the fruit. A rule of thumb is that the higher the sugar levels in the fruit, the lower the freezing point (Gross et al., 2016).
| Storage conditions (°F) | Storage conditions (°C) | Chilling injury | Storage life (days) |
|---|---|---|---|
| 30.5–32 | -1–0 | no | 21–30 |
| 36–46 | 2.2–7.7 | yes | 5–7 |
| 50 | 10 | no | 21–30 |
| 66–68 | 19–20 | no | 3–5 |
Relative humidity is an important factor that affects storage life and eating quality. Peaches have a high concentration of water (88%) and should be stored in high-humidity conditions in order to retain their quality and not get dehydrated. The recommended postharvest relative humidity levels are between 90%–95%.
Preconditioning is a postharvest method used by some western shippers where fruit is held at 68 °F (20 °C) for 24–48 hr after packing but before cold storage. The delay in cooling the fruit allows the ripening process to begin before shipment and helps decrease internal breakdown development, which can be caused by improper storage temperature. Additionally, the practice of preconditioning allows peaches to be harvested firm enough to withstand the physical stresses of packing and shipping, and ripens them enough to reduce internal disorders. It is important to consider that weight loss and fruit softening could occur when preconditioning peach fruit (Moore & Farcuh, 2022).
References
Belisle, C., Phan, U. T. X., Adhikari, K., & Chavez, D. J. (2018). A fruit quality survey of peach cultivars grown in the southeastern United States. HortTechnology, 28(2), 189–201. http://dx.doi.org/10.21273/HORTTECH03870-17
Cai, Y., & Farcuh, M. (2021, July 20). Determining peach fruit maturity. University of Maryland Extension. https://extension.umd.edu/resource/determining-peach-fruit-maturity
Cirilli, M., Bassi, D., & Ciacciulli, A. (2016). Sugars in peach fruit: A breeding perspective. Horticulture Research, 3, 15067. https://doi.org/10.1038/hortres.2015.67
Crisosto, C. H., & Crisosto, G. M. (2005). Relationship between ripe soluble solids concentration (RSSC) and consumer acceptance of high and low acid melting flesh peach and nectarine (Prunus persica (L.) Batsch) cultivars. Postharvest Biology and Technology, 38(3), 239–246. https://doi.org/10.1016/j.postharvbio.2005.07.007
Crisosto, C. H., & Mitchell, F. G. (2002). Postharvest handling systems: Stone fruits. In A. A. Kader (Ed.), Postharvest Technology of Horticultural Crops (3rd ed., pp. 345–352). UC Davis Department of Plant Sciences. https://postharvest.ucdavis.edu/publication/postharvest-technology-horticultural-crops-3rd-edition
Crisosto, C. H., Mitchell, F. G., & Ju, Z. (1999). Susceptibility to chilling injury of peach, nectarine, and plum cultivars grown in California. HortScience, 34(6), 1116–1118. https://doi.org/10.21273/HORTSCI.34.6.1116
Gross, K. C., Wang, C. Y., & Saltveit, M. (Eds). (2016, February). The commercial storage of fruits, vegetables, and florist and nursery stocks (Agriculture Handbook No. 66). U.S. Department of Agriculture Agricultural Research Service. https://www.ars.usda.gov/is/np/CommercialStorage/CommercialStorage.pdf
Huang, X., Wang, H., Luo, W., Xue, S., Hayat, F., & Gao, Z. (2021). Prediction of loquat soluble solids and titratable acid content using fruit mineral elements by artificial neural network and multiple linear regression. Scientia Horticulturae, 278, 109873. https://doi.org/10.1016/j.scienta.2020.109873
Iezzoni, A. (2019, November 14). Peach fruit texture. The Extension Foundation. https://plant-breeding-genomics.extension.org/peach-fruit-texture/
Jobling, J. (2000). Practical solutions for temperature management. Good Fruit and Vegetables, 10, 12.
Kao, M.-W. S., Brecht, J. K., Williamson, J. G., & Huber, D. J. (2012). Ripening development and quality of melting and non-melting flesh peach cultivars. HortScience, 47(7), 879–885. https://doi.org/10.21273/HORTSCI.47.7.879
Lurie, S., & Crisosto, C. H. (2005). Chilling injury in peach and nectarine. Postharvest Biology and Technology, 37(3). https://doi.org/10.1016/j.postharvbio.2005.04.012
Minas, I. S., Anthony, B. M., Pieper, J. R., & Sterle, D. G. (2023, February). Large-scale and accurate non-destructive visual to near infrared spectroscopy-based assessment of the effect of rootstock on peach fruit internal quality. European Journal of Agronomy, 143, 126706. https://doi.org/10.1016/j.eja.2022.126706
Mitcham, E. (2023, October 4). Nectarine & peach: Recommendations for maintaining postharvest quality [Produce Fact Sheet]. UC Davis Postharvest Research and Extension Center. https://postharvest.ucdavis.edu/produce-facts-sheets/peach
Moore, K., & Farcuh, M. (2022). What is chilling injury in peaches, what causes it, and how can you manage it? (Publication No. FS-1141). University of Maryland Extension. https://extension.umd.edu/resource/what-chilling-injury-peaches-what-causes-it-and-how-can-you-manage-it-fs-1141
National Agricultural Statistics Service. (2024, May 8). Southern region news release: Noncitrus fruits and nuts [Press release]. U.S. Department of Agriculture. https://www.nass.usda.gov/Statistics_by_State/Regional_Office/Southern/includes/Publications/Crop_Releases/Fruit_Production/NoncitrusFruitNut2024.pdf
Scalisi, A., Pelliccia, D., & O’Connell, M. G. (2020). Maturity prediction in yellow peach (Prunus persica L.) cultivars using a fluorescence spectrometer. Sensors, 20(22), 6555. https://doi.org/10.3390/s20226555
Shane, W. (2011, July 25). Monitoring peach and nectarine ripening. Michigan State University Extension. https://www.canr.msu.edu/news/monitoring_peach_and_nectarine_ripening
Spadoni, A., Cameldi, I., Noferini, M., Bonora, E., Costa, G., & Mari, M. (2016). An innovative use of DA-Meter for peach fruit postharvest management. Scientia Horticulturae, 201, 140–144. https://doi.org/10.1016/j.scienta.2016.01.041
Taylor, K. C., & Rushing, J. W. (2012). Harvesting and handling peaches. In Southeastern peach growers’ handbook (pp. 107–126). University of Georgia College of Agricultural and Environmental Sciences.
Wang, L. (2009). Heritable pleiotropy of glabrous and saucer shape gene loci from peach and their breeding value. CABI. https://www.cabdirect.org/cabdirect/abstract/20093359009
Warnert, J. (2005). Key to delicious tree fruit is keeping it out of the "killing zone." University of California Cooperative Extension.
Wu, H., Xu, Y., Wang, H., Miao, Y., Li, C., Zhao, R., Shi, X., & Wang, B. (2022). Physicochemical characteristics, antioxidant activities, and aroma compound analysis of seven peach cultivars (Prunus persica L. Batsch) in Shihezi, Xinjiang. Foods, 11(19), 2944. https://doi.org/10.3390/foods11192944
Zhang, B., Peng, B., Zhang, C., Song, Z., & Ma, R. (2017, May 15). Determination of fruit maturity and its prediction model based on the pericarp index of absorbance difference (IAD) for peaches. PLOS ONE, 12(5), e0177511. https://doi.org/10.1371/journal.pone.0177511
Ziosi, V., Noferini, M., Fiori, G., Tadiello, A, Trainotti, L., Casadoro, G., & Costa, G. (2008). A new index based on vis spectroscopy to characterize the progression of ripening in peach fruit. Postharvest Biology and Technology, 49(3), 319–329. https://doi.org/10.1016/j.postharvbio.2008.01.017
Status and Revision History
In Review on Aug 02, 2024
Published on Aug 22, 2024


























































