Mastitis is an economically important disease of dairy cows because it reduces the quantity as well as the quality of milk produced, and as a consequence, lowers producer profits. Control of this disease is based on the following recommended milking procedures: pre- and post-milking teat sanitization, the use of single-service paper or cloth towels to dry udders, and proper milking machine use; prompt antibiotic treatment of clinical cases; dry cow therapy; proper nutrition; and maintaining a clean and dry environment. In addition, vaccination against this disease has been recommended to prevent new infections, thereby eliminating the use of antibiotics in food animals.
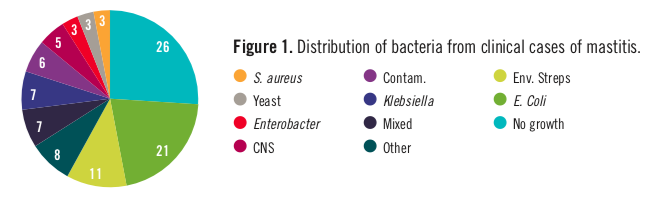
The purpose of vaccinating against mastitis-causing bacteria is to stimulate the cow’s immune system to protect it against subsequent infection or disease. For example, vaccination may increase circulating antibodies in the blood stream against certain mastitis pathogens to prevent or limit bacterial growth after invasion into a mammary quarter. The resulting enhanced immunity may also minimize pathogen damage to milk-producing tissues, modify the inflammatory response, promote tissue repair, and reduce the clinical expression of disease. A list of bacteria isolated from clinical cases of mastitis is shown in Figure 1. Most cases are caused by coliforms such as Escherichia coli (21%), Klebsiella (7%), and Enterobacter (3%) as a group, followed by the environmental streps (11%), coagulase-negative staphylococci (or CNS, 3%), and Staphylococcus aureus (3%), which are isolated less frequently.
Progress has been made in efforts to develop vaccines for preventing both contagious and environmental mastitis. There are commercial mastitis vaccines currently available for E. coli, S. aureus, and Mycoplasma bovis, and several experimental vaccines based on these three pathogens have been the focus of the pharmaceutical industry and academic institutions for many years. Far less information is available on streptococcal vaccines, and there are currently none commercially available. According the latest National Animal Health Monitoring System survey in 2014, 18.7% of U.S. dairy operations used some type of mastitis vaccine to control this disease, and use increased as herd size increased (Table 1).
Table 1. Percentage of dairy operations that use vaccines to control mastitis, by herd size.
|
Very small (<30) |
Small (30-99) |
Medium (100-99) |
Large (500+) |
All operations | |
| Vaccine | Percentage using vaccination | ||||
|
E. Coli |
2.4 | 12.4 | 27.1 | 50.8 | 18.1 |
| Staphylococcus aureus | 1.5 | 1.9 | 0.8 | 0.3 | 1.4 |
| Mycoplasma | 0.0 | 0.0 | 0.0 | 0.3 | 0.0 |
| Any | 3.9 | 13.0 | 27.5 | 50.9 | 18.7 |
Coliform vaccines, the most popular on the market:
The most success in vaccinating cows against mastitis has been realized with gram-negative common core vaccines, which means that the vaccine is meant to target a common portion of many gram-negative pathogens. Bacterins (killed or attenuated bacterial preparations) formulated against coliforms (e.g., Escherichia, Klebsiella, and Enterobacter) have been developed because the proportion of mastitis caused by environmental bacteria, i.e., coliforms, has increased in many herds. This may be due to: (1) the trend for low somatic cell count (SCC) milk; (2) an increase in cow susceptibility to coliform mastitis; and (3) higher density housing, which increases exposure to environmental pathogens. In addition, common herd health practices such as teat dipping and antibiotic therapy are not effective in controlling coliform mastitis, primarily because of the continuous exposure to these pathogens within the cow’s environment. Coliform mastitis may range in severity from subclinical infections to peracute clinical cases. A high proportion of clinical cases occurs within the first three months of lactation, mainly during the first two weeks after calving, causing marked losses in milk production, e.g., $100 to $300 per clinical coliform case. Therefore, it is important that the dairy farmer avoid the disease or minimize the risk of infection in the herd.
How widespread is coliform vaccine use? The National Animal Health Monitoring System estimates that coliform mastitis vaccines were used on approximately 18% of U.S. dairy farms, and use increases as herd size increases (Table 1). For example, coliform vaccines are used in over 50% of large operations milking 500+ cows, whereas only 2.4% of very small farms (<30 cows) use the vaccine. An examination of the percentage of operations that administer mastitis vaccines by U.S. region (Table 2) indicates that E. coli vaccines are more popular in the West than the East (35.7% vs. 16.5%), which is most likely associated with greater herd sizes in the West.
Table 2. Percentage of operations that administered mastitis vaccines by region.
| Region | ||
| Vaccine | West | East |
| E. Coli | 35.7 | 16.5 |
| Staphylococcus aureus | 0.3 | 1.5 |
| Mycoplasma | 0.0 | 0.0 |
| Any | 35.9 | 17.3 |
Control of coliform mastitis has been made possible through the development of mutant gram-negative bacteria. Vaccines used to combat gram-negative pathogens focus on using the mutant gram-negative core antigen, which lacks the chains that protect the lipopolysaccharides of gram-negative pathogens. This characteristic is important because the antibodies produced by the vaccinated animals are specific to the exposed lipopolysaccharides of all gram-negative organisms whether they are of the genus Escherichia, Klebsiella, or Enterobacter. Thus, such vaccines stimulate the production of antibodies against common core antigens in the bacterial cell wall that are cross-protective against a wide variety of gram-negative microorganisms. Three such vaccines are described below.
ENVIRACORTM J-5 Vaccine: One of the vaccine products is an Escherichia coli J5 mutant bacterin administered subcutaneously at drying off, 30 days later, and again within 14 days of calving (E. coli J5 Strain, Zoetis, Parsippany, New Jersey). Use of this strain of bacteria (Escherichia coli J5 ) in coliform vaccine formulation is unique because it stimulates antibody production against a wide variety of coliform bacteria, including Klebsiella and Enterobacter species.
Following initial observations that cattle with low naturally-occurring blood antibodies against E. coli J5 experienced a fivefold increase in clinical coliform mastitis, researchers in California investigated the efficacy of vaccination in reducing the incidence of clinical coliform mastitis. Vaccinated animals received a total of three subcutaneous injections: 1) the first day of drying off, 2) 28 days after being dried off, and 3) within 14 days of calving. Results showed that during the subsequent 100 days of lactation, the incidence of clinical cases of coliform mastitis was reduced by 80% in animals that were vaccinated.
This same vaccine was subsequently evaluated by researchers in Ohio where vaccinations were given subcutaneously at drying off, 30 days later, and two days after calving. Compared with controls, vaccinated cows exhibited fewer bacteria in milk and lower rectal temperatures following an E. coli mastitis challenge at 30 days into lactation. In addition, unvaccinated animals experienced greater milk yield and dry matter intake (DMI) depression compared to vaccinated animals. Antibodies in the blood and milk were also higher in vaccinated than control cows. It was concluded that vaccination with the E. coli J5 bacterin did not prevent infections but did reduce severity of clinical signs following intramammary challenge with E. coli.
This vaccine was then field tested for 2.5 years in a commercial herd under natural exposure conditions and compared with a control group. A total of 67% of gram-negative bacterial infections present at calving in control cows became clinical during the first 90 days of lactation compared with only 20% in vaccinated cows. Thus, the vaccine was over three times more efficacious in reducing clinical mastitis caused by coliform mastitis pathogens compared to unvaccinated animals. New coliform infections, along with severity of clinical mastitis, were also decreased in first lactation heifers.
A partial budget analysis of on-farm implementation of an E. coli J5 vaccination program conducted in 1991 demonstrated that the use of the vaccine on all cows in a herd was profitable when incidence of clinical coliform mastitis exceeded 1%. Using such a program would yield a $57 profit per cow per lactation, and the return on the investment into the vaccine would be approximately 1,700%.
J-VAC® Escherichia coli Bacterin-Toxoid: Another gram-negative vaccine based on the Escherichia coli mutant strain is the J-VAC®, manufactured by Merial Ltd. of Duluth, Georgia. Studies on this bacterin indicate that it is approximately 60% effective in reducing expression of clinical coliform mastitis. In addition, unvaccinated animals experienced increased milk production depression normally encountered with endotoxemia compared to animals vaccinated with J-VAC®. Following label instructions, this vaccine is administered subcutaneously or intramuscularly in the neck at drying off and then cows are boosted two to four weeks later. The injection regimen is followed after each lactation to provide adequate antibody levels during the periparturient period and during early lactation to help provide protection against clinical coliform infections.
ENDOVAC-Dairy ® : Another USDA-licensed coliform vaccine is a bacterin-toxoid formulated from a Re-17 mutant of Salmonella typhimurium (ENDOVAC-Bovi®; Immvac Inc., Columbia, Missouri) administered during the dry period and cows are boosted two or three weeks later. It works similarly to vaccines formulated with the E. coli J5 in stimulating protection against common gram-negative core antigens. In addition, the toxoid component is believed to stimulate immune cells in the cow’s body to enhance antibody production to Salmonella typhimurium Re-17.
A field trial to test this vaccine in Arizona utilized cows immunized intramuscularly at dry-off and again two to three weeks prepartum and compared them to unvaccinated controls. Data collected over the first five months of lactation showed a 42% reduction in clinical cases of coliform mastitis in vaccinates compared with controls, and a 67% reduction in repeat episodes. In addition, the mortality rate for cows with clinical coliform mastitis was 61% lower in vaccinated cows. Likewise, the culling or removal rate was 61% lower in vaccinates compared with controls.
S. aureus vaccines are used less frequently: Early efficacy studies on the only commercial S. aureus vaccine in the U.S. (Lysigin®, Boehringer Ingelheim Vetmedica, Inc., St. Joseph, Missouri) suggests that it will increase the spontaneous cure rate (no intervention from farm or veterinarian) against S. aureus IMI and lower SCC, but does not prevent new IMI in adult cows. Research conducted over the past 15 years has demonstrated that several experimental S. aureus vaccines, as well as one commercial vaccine, can reduce the new infection rate in dairy heifers. For example, a S. aureus vaccine formulated to stimulate antibodies against two important components of S. aureus (pseudocapsule and alpha toxin) was evaluated in heifers in New York. At four and two weeks prior to calving, heifers were given subcutaneous injections into the supramammary lymph node of the mammary gland, and after calving, heifers were challenged with S. aureus. Vaccinates demonstrated a 52% reduction in the development of new IMI post-challenge. In addition, 64% of IMI in control cows became chronic compared with only 12% in vaccinates. Subsequently, a field trial in Norway evaluating a S. aureus vaccine demonstrated that vaccinated heifers injected subcutaneously in the area of the supramammary lymph node of the mammary gland at eight and two weeks before calving showed a 46% reduction in new IMI during the subsequent lactation compared with controls. In Argentina, a vaccine formulation was evaluated in bred heifers vaccinated intramuscularly at eight and four weeks and one and five weeks postpartum. This immunization program demonstrated that the frequency of new S. aureus IMI was reduced by 66% in vaccinated animals.
In view of the above studies showing the success of experimental vaccines in heifers, researchers in Louisiana evaluated the commercial S. aureus vaccine, Lysigin®, in young dairy animals. At six months of age, 35 Jersey heifers in a research herd were vaccinated per label instructions intramuscularly, 14 days later, and at six-month intervals until calving. Results demonstrated that the rate of new IMI at freshening was reduced 44.7% and SCC reduced 50% in vaccinates compared with the 35 control heifers. Serum samples demonstrated that anti- staphylococcal antibody titers remained higher in vaccinated heifers compared to controls throughout the study suggesting an enhanced ability for heifers to combat S. aureus infections.
Subsequently, Missouri researchers compared two experimental S. aureus formulations with the commercially available S. aureus vaccine, Lysigin® in heifers. Animals were vaccinated twice, 28 days apart, during late gestation. After calving, they were challenged by intramammary infusion with S. aureus in early lactation. All quarters became infected with S. aureus, and at the end of the study, there were no differences in S. aureus clearance rates, SCC, or milk yields.
In contrast to the Missouri work, a trial in Virginia found beneficial results in using Lysigin® to manage S. aureus mastitis in heifers. Using 106 Holstein heifers six to 18 months of age in a commercial herd, 53 animals were vaccinated intramuscularly using a 5-milliliter dose, and 53 served as unvaccinated controls; 14 days later, the vaccinated group was boosted with Lysigin® and at 6-month intervals thereafter through calving. Vaccine efficacy data showed that the percentage of heifers with S. aureus IMI at freshening was lower in vaccinates (13.3%) compared with controls (34.0%)—a reduction of 60.9%. Also, SCC collected during the first week of lactation were lower in vaccinates compared with controls (287,000 vs. 522,000/milliliter).
Thus, the use of experimental and commercially available S. aureus vaccines may be used to prevent new infections when used in heifers, though studies have shown varied results. Efficacy has been shown to range between 44% to 66%, and this prevention strategy may represent a major control mechanism for managing S. aureus in the future, especially as new antigens and adjuvants are added to vaccine preparations to enhance their effectiveness.
The National Animal Health Monitoring System estimates that S. aureus vaccines are used on approximately 1.4% of United States dairy farms (Table 1), and tend to be more popular on very small (1.5%) and small (1.9%) herds than medium (0.8%) and large (0.3%) herds. In addition, S. aureus vaccines are more likely to be used in the eastern US (1.5%) than the western US (0.3%) (Table 2).
Autogenous S. aureus vaccines: Autogenous vaccines are those formulations that are prepared from cultures of microorganisms (specific strains of S. aureus) obtained from individual cows with mastitis and then used to immunize or protect other animals in that herd against further udder infection with the same strain of S. aureus. There is some evidence that the use of autogenous S. aureus vaccines can significantly elevate S. aureus blood antibody titers and reduce subclinical and clinical mastitis in vaccinated animals compared with unvaccinated control cows. In one study, use of an autogenous vaccine composed of three S. aureus strains resulted in 70% protection from infection and a reduction in severity of clinical mastitis in cows experimentally challenged with S. aureus.
Mycoplasma vaccines: AgriLabs of St. Joseph, Missouri, introduced Mycomune® bacterin, the first USDA-licensed vaccine for the prevention of Mycoplasma mastitis caused by Mycoplasma bovis. A commercially available Mycoplasma bovis bacterin was developed that is injected subcutaneously by giving two doses at two- to four-week intervals during the prepartum period and the third dose two to three weeks prepartum, which claims to reduce the incidence and severity of Mycoplasma mastitis. In a California trial conducted in 2002, blood antibodies increased fourfold and milk antibodies tenfold compared to controls after the third vaccination given two to three weeks prepartum. Importantly, vaccination was shown to prevent new infections after experimental challenge with M. bovis in early lactation, minimize culling of cows, and reduce positive bulk tank cultures. Additionally, milk production was maintained in vaccinates but decreased markedly in controls.
Experimental autogenous Mycoplasma vaccines have also been developed using specific strains from problem herds in the vaccine formulation. Preliminary data suggest that new infections are prevented, but such data are largely based on testimonials. To date, no peer-reviewed studies are available, so efficacies of commercial and autogenous vaccine attempts have not been established. One problem is that the surface antigens (molecules) of Mycoplasma organisms are highly variable and can change over time in response to host or environmental conditions. This makes immunization difficult because of the rapid change of antigens. The National Animal Health Monitoring System estimates that, currently, Mycoplasma mastitis vaccines are used on 0% of U.S. dairy farms.
For the 18.7% of dairy operations that administered vaccines to cows, the average cost of vaccinations per cow, by herd size and by region, is shown in Table 3. Costs ranged from $3.92 for medium-sized herds to $6.58 for small herds; from $4.48 for Western herds to $5.61 for Eastern herds. The average of all operations was $5.41.
Table 3. Average cost of vaccination per cow, by herd size and by region
| Operation Average Cost Per Cow ($) | |||||
| Herd size | Region | ||||
|
Small (30-99) Avg. |
Medium (100-499) Avg. |
Large (500+) Avg. |
West Avg. |
East Avg. |
All operations Avg. |
| 6.58 | 3.92 | 5.46 | 4.48 | 5.61 | 5.41 |
Strep. uberis vaccines: There are currently no commercially available vaccines directed against the environmental streptococci, such as Strep. uberis, despite its prevalence during the prepartum period. However, many private and academic institutions are examining the development of such vaccines, and when one becomes available for efficacy evaluation, a test model must be in place to adequately evaluate the product. For example, after animal safety has been established, such a model would include: a) immunized and control heifers or cows; b) monitoring the new infection rate with the organism against which the vaccine was developed; and c) determining if the vaccinated animals exhibited a lower rate of infection than unvaccinated controls. This model would rely on the natural infection rate in a group of animals, which may be rather low, and would require quite a lot of time to acquire a sufficient number of infections. To increase the rate of new IMI, and therefore reduce the amount of time for product assessment, experimental challenge models have been used.
Mastitis vaccines – the bottom line: Because of universal exposure to manure, which contains E. coli and other gram-negative bacteria, as well as the requirement to maintain SCC as low as possible, all cows should be vaccinated with one of the coliform vaccines available on the market. These vaccines have been proven to significantly reduce clinical coliform mastitis, and have been shown to be profitable when incidence of clinical coliform mastitis exceeds 1% of the milking cows. The one commercially available S. aureus vaccine may be beneficial in enhancing the ability of a cow to cure herself of Staph. mastitis and in lowering the SCC, but is generally not recommended for adult cows. A list of commercially available mastitis vaccines is found in Table 4.
Table 4. Summary of mastitis vaccine by type, trade name, manufacturer, and administration.
| Vaccine type | Trade name | ,Manufacturer | Administration |
| Coliform |
ENVIRACORTM J-5 |
Zoetis |
3 shots: At 7 and 8 months of gestation and within 2 weeks of calving; 5cc SC or IM/shot |
|
J-VAC® |
Merial |
2 shots: At dry-off and a boost 1 to 3 weeks prepartum; 2cc SC or IM/shot |
|
|
ENDOVAC-Dairy® |
Immvac Inc. |
2 shots: During dry period and boost 2 or 3 weeks later; 2cc (IM)/shot |
|
| S. aureus |
Lysigin® |
Boehringer Ingelheim Vetmedica, Inc. |
3 shots: 5cc IM; boost 14 days later, and at 5-6 months |
| Mycoplasma |
Mycomune® |
AgriLabs |
3 shots: First 2 are 2 weeks apart followed by a last shot 2 to 3 weeks prepartum; 2cc SC/shot |
Status and Revision History
Published with Full Review on Jan 16, 2019
Published with Full Review on Jul 21, 2022


























































