Disease-causing microorganisms, or “pathogens,” in household waters primarily include bacteria, viruses, and protozoa. These pathogens pose a serious threat to human health because their impact on the body can be almost immediate. You may have seen examples of serious outbreaks of waterborne diseases in the news media from time to time. For example, according to the Centers for Disease Control (CDC) and Prevention Waterborne Disease and Outbreak Surveillance System, from 2011 to 2012, 32 microbial-associated disease outbreaks in drinking water were reported in the U.S., accounting for at least 431 cases of illness, 102 hospitalizations, and 14 deaths. This data can be found at http://www.cdc.gov/healthywater/surveillance/index.html.
The most commonly identified reason for these outbreaks is the bacterium Legionella spreading from within building plumbing systems (accounting for 66% of the aforementioned cases) and untreated groundwater (13% of the cases). Therefore, disinfection is considered important to protect human health in most water treatment systems. This publication provides information on the various disinfection options to consider before buying disinfection equipment in the following order:
- Chlorination
- UV radiation
- Ozonation
- Pasteurization
- Distillation
- Disinfection equipment
Test your water before implementing these processes, and with the help of a water specialist, determine whether you have water quality problems that should be addressed by installing an appropriate water treatment system.
Chlorination
A common and relatively inexpensive method of disinfecting water is chlorination, where a solution of chlorine or hypochlorite is added to the water. The method kills disease-causing microorganisms including bacteria and certain viruses but does not kill the protozoa Cryptosporidium, Giardia, and some others. The process also removes some bad odors,
Water can be chlorinated in two ways:
1. Shock chlorination is when a strong chlorine solution is added into a well or pumped through the plumbing system to kill microorganisms on a one-time basis. Shock chlorination is usually recommended when a well is constructed, repaired, or a new pump is installed to kill bacteria that may be present on the pipes or installation equipment. It is also recommended if a well is contaminated due to a faulty well cap or seal. More information about using shock chlorination in well water can be found in UGA Extension Circular 858-4, Disinfecting Your Well Water: Shock Chlorination.
2. Continuous chlorination is a process where chlorine is added continuously to the household water through a chemical feed pump. This system is required when the source of the bacteria in the water cannot be eliminated and recurrent bacterial contamination is encountered even after several shock chlorination treatments. Continuous chlorination is a point-of-entry treatment method that can offer residual disinfection throughout the household water distribution system. Almost all public (city or county) water systems use this system, often in combination with others.
Contaminants removed: Bacteria, some viruses, iron, manganese, and hydrogen sulfide
System cost (continuous chlorinator): On average, $900 for a family of four
How does chlorination work?
In the proper concentrations and under adequate exposure/contact time, chlorine is an excellent disinfectant for bacteria and certain viruses. The contact time is the time available to complete the reaction between the chlorine and untreated water. A longer contact time results in more effective disinfection. For example, a higher chlorine concentration requires a shorter contact time and vice versa. However, even with adequate concentration and contact time, the effectiveness of chlorination depends on various other factors, including water temperature, water pH, and water turbidity, or haziness caused by suspended particles in the water. Chlorination is more effective at a high temperature and at pH 4.0-7.0. For every 18°F drop in water temperature, there could be as much as a 50% reduction in the efficacy of disinfection, doubling the required contact time. Thus, with a given chlorine concentration, you would need longer contact time in winter than in summer to properly disinfect the water.
Particulate matter in water decreases the effectiveness of chlorination because microorganisms may “hide” behind particles and avoid disinfection. Also, chlorine readily combines with other components dissolved in water, including iron, manganese, hydrogen sulfide, organic matter, ammonia, and organic color such as that from decaying peat moss which may reduce the effectiveness of the disinfection. To overcome this, it isimportant to add sufficient chlorine to meet the chlorine demand and provide residual disinfection. “Chlorine demand” is a term used to describe the total added chlorine subtracted by residual, inactive chlorine. The added chlorine that does not combine with other components is available to disinfect the water, commonly called the “free chlorine residual.” According to the CDC, treated water should contain a free chlorine level of at least 0.5 mg/liter to be microbiologically safe and no more than 2.0 mg/L to make sure that the water does not have an unpleasant taste or odor. Free chlorine residual can be measured on-site using a chlorine test kit.
See http://aesl.ces.uga.edu/Water/ChlorineTest.pdf for more information on chlorine testing.
Types of chlorination
The different types of chlorine disinfection are batch disinfection, simple chlorination, and superchlorination followed by dechlorination. Chlorination types use various amounts of chlorine.
Batch chlorination is especially useful for cisterns, holding tanks, or during emergencies or other special situations. Three tanks, each capable of holding a two to three days’ water supply, are alternately filled to allow adequate contact time and ensure satisfactory disinfection. The water is treated and used as needed.
Simple chlorination maintains a low level (0.3 to 0.5 mg/liter) of free chlorine residual throughout the water/plumbing system. The residual should be measured at the faucet farthest from the chlorine source. Continuous chlorinator equipment is used for this type of chlorination.
Superchlorination followed by dechlorination (chlorine removal) is an option when the necessary contact time is unattainable. Superchlorination produces a higher free chlorine residual of 3.0 to 5.0 mg/liter. At
this concentration, contact time as short as five minutes may be sufficient to achieve expected disinfection. Superchlorinated water has a strong chlorine smell and taste that is removed with an activated carbon filter following chlorine treatment. If the water contains dissolved iron, manganese, or sulfur, it may be necessary to install a sediment filter before the activated carbon unit to prevent clogging of the activated carbon filter.
Equipment options
Various kinds of injection devices and pumps are available to chlorinate a private water supply, such as water from a private well. The injection device should operate only when the water pump is on, and the water pump should shut off if the chlorinator fails or if the chlorine supply runs out.
Two formulations of chlorine are available for chlorination: (1) dry powder or pellet (calcium hypochlorite) or (2) liquid (sodium hypochlorite). Chlorine solutions from powders should not be prepared in large quantities at a time for long-term use; it should be prepared as needed because the strength of the solution decreases gradually after mixing. To avoid hardness deposits on equipment, manufacturers recommend using softened or distilled water when mixing chlorine solutions. The effectiveness of chlorination depends on the amount of chlorine used. The amount is controlled by adjusting the equipment or by changing the amount of chlorine added for batch disinfection.
Maintenance
The following maintenance may apply to a chlorination unit:
-
Checking for loose, worn, missing, or broken parts
-
Lubricating the entire system semiannually
-
Cleaning all surfaces showing corrosion
-
Refilling chlorine supplies
-
Cleaning any clogged orifices
Be sure to unplug any power cords before maintenance. Always follow the maintenance guidelines laid out in the manufacturer’s manual and keep record of maintenance activities.
Special considerations
Both solid and liquid forms of chlorine can irritate the skin, are poisonous in concentrated form, and must be handled and stored carefully. Chlorine tablets should be stored in a dry location. Both liquid and solid formulations should be stored in their original labeled containers, away from children and animals.
Excess chlorine can impart an unpleasant taste and smell to the treated water. Also, if the water being treated contains organic molecules, the formation of hazardous chlorinated organic compounds like trihalomethanes is possible. Trihalomethanes result from the reaction of organic compounds in water with chlorine. To reduce the formation of these compounds, an organic filter should be considered prior to the chlorination step if high concentrations of organic and inorganic matter are present in the water. Using an activated carbon filter after chlorination will remove excess chlorine and limited amounts of any chlorine compounds formed. Using a carbon filter after chlorination will remove any excess chlorine and chlorine-based chemicals that may form.
In a properly operating chlorination system, the low level of residual chlorine that remains in the household drinking water will not harm the septic system. High levels of chlorine, caused by issues like an improperly adjusted or malfunctioning chlorinator, may be a problem for the desirable bacteria population in the septic system and could result in the unpleasant taste and smell of excessive chlorine.
A common household chlorination system (Figure 1) consists of a chlorine tank, a chlorine injector to dose the incoming water with the right amount of chlorine, a pressure tank to maintain system pressure, a contact tank to give the chlorine time to work, and optional post-processing activated carbon filter to remove residual chlorine and chlorination byproducts (e.g., harmful trihalomethanes) from the water.
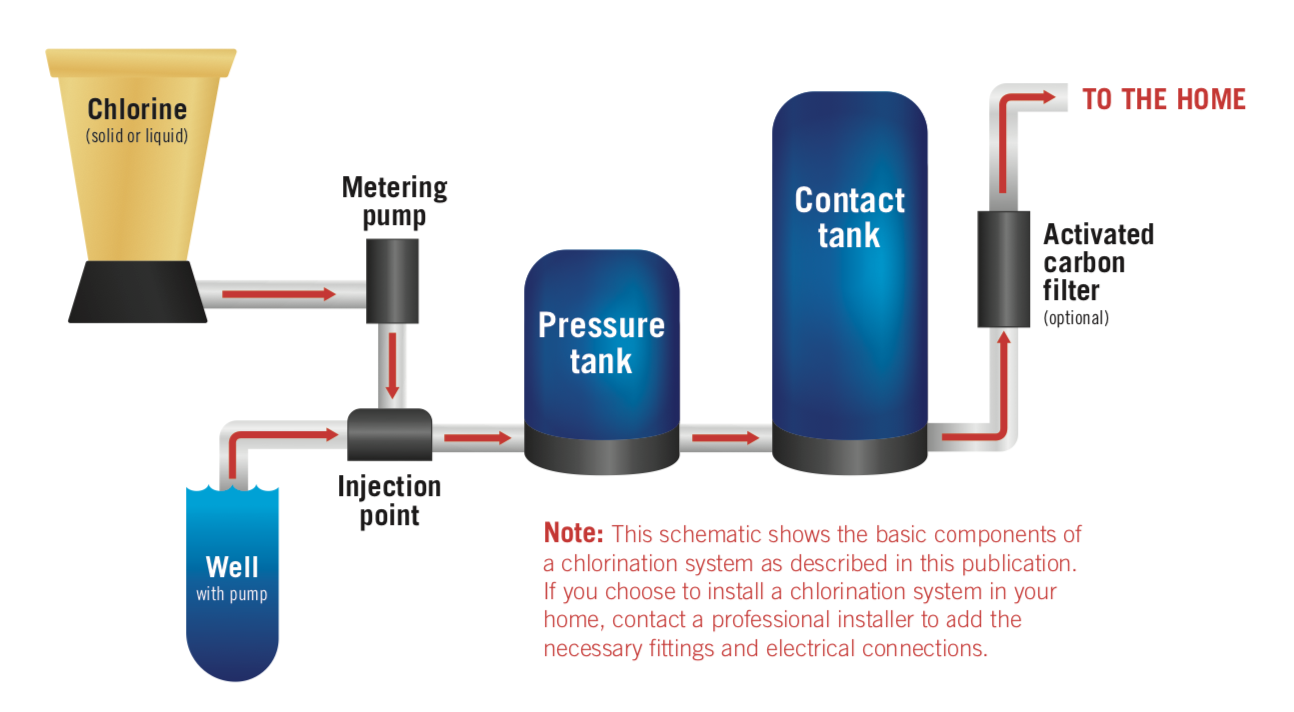
Figure 1. Diagram of a common household chlorination system.
How chlorination helps to remove iron, manganese, and hydrogen sulfide
Iron and manganese do not affect health, but they can make water bitter, stain laundry and fixtures, and discolor water. Refer to http://aesl.ces.uga.edu/publications/watercirc/IronManganese.pdf?2 for more information. The U.S. Environmental Protection Agency (EPA) has given a secondary drinking water standard (https://www.epa.gov/dwstandardsregulations) of 0.3 milligrams per liter for iron and 0.05 milligrams per liter for manganese. Hydrogen sulfide (see UGA Extension Circular 858-8) is a nuisance contaminant that gives water an odor akin to rotten eggs.
Soluble iron and manganese that naturally occur in water are in a dissolved and colorless form. Chlorination can oxidize iron into insoluble red-brown, manganese into black, and hydrogen sulfide into yellow particles. These insoluble particles can be removed by mechanical filtration. A pH between 6 and 8 is best for iron or hydrogen sulfide removal by chlorination. Manganese removal is most effective when the pH is greater than 9.5. A certain amount of contact time between the contaminant and the chlorine is required. Contact usually occurs in the system’s pressure tank, although water might not remain in the tank long enough for complete oxidation, which is why a contact tank may be used.
An alternative to using a tank to achieve an adequate contact time is super chlorination, which adds much more chlorine than is necessary. This procedure results in more efficient oxidation, but the excess chlorine must be removed with an activated carbon filter.
Ultraviolet light
Ultraviolet (UV) light has been used to disinfect water supplies for over 75 years, but home UV systems have only become available relatively recently. This type of water treatment uses a low-pressure mercury arc lamp that emits UV light to kill pathogens in the water. The principal advantage to UV treatment is that it disinfects water without using any chemicals. Furthermore, some waterborne disease-causing microorganisms are resistant to chlorine. These chlorine-resistant microorganisms include viruses, parasites, and bacteria that can cause hepatitis, gastroenteritis, cryptosporidiosis, and Legionnaires’ disease. UV treatment is especially useful in this regard.
Although UV treatment is an effective disinfectant, disinfection only occurs within the unit. Disinfection does not occur beyond the treatment unit to kill microorganisms that survived or were introduced to the system after UV treatment. If residual disinfection is necessary, chlorination may be necessary in addition to or as an alternative to UV treatment.
UV light kills bacteria, viruses, and some cysts. It does not kill certain organisms such as Giardia lamblia cysts or Cryptosporidium parvum oocysts, which must be removed by filtration or distillation. UV is not recommended if the untreated water has a coliform content exceeding 1,000 total coliforms or 100 fecal coliforms per 100 milliliters.
The UV unit can either be a point-of-entry (POE) system, treating all the water entering the house or a point-of-use (POU) device, treating water from a single tap, as a final disinfection method. UV systems may have a flow rate capacity of as low as 0.5 gallon per minute (GPM) for the POU unit to as high as several hundred GPM for the POE units. Household water requirements dictate the size of the unit needed. A 10 GPM flow rate is adequate for most homes.
System cost: $250 (for under the sink) to more than $1,000 (for the whole house)
General system structure
A typical UV treatment device consists of a low-pressure mercury lamp, housed in a cylindrical chamber, that most often produces UV light using a wavelength of 254 nanometers (nm). Lamps vary in length from 12 to 48 inches and thus vary in energy output. The lamp is encased in a quartz glass sleeve to prevent water from contacting the lamp. The quartz sleeve also helps keep the lamp at an ideal operating temperature of 104°F. Approximately 95% of the UV light passes through this glass sleeve and into the untreated water. Untreated water either flows in a thin film over the sleeve, or it flows through quartz glass tubing that spirals around the lamp. The spiral design allows for a longer contact time between the UV light and the untreated water.
How does a UV system work?
Water to be treated is exposed to UV light. The UV light kills or deactivates pathogens by destroying their DNA. Bacteria are killed with relatively low 20 mJ/cm2 radiation, but viruses typically require 45 mJ/cm2 to be killed, while cysts and worms are unaffected. The light is destructive to microorganisms but safe for humans, although you should not look at UV lamps directly, as doing so can cause eye damage. UV light’s killing capacity depends on the intensity of the UV light, the contact time, and the amount of suspended solid particles in the water as discussed below in section 2.3. To be effective, the system typically requires only a few seconds of exposure and it does not add any taste or odor to the treated water. The depth of light penetration into water is no more than 2 to 3 inches.
System efficiency and maintenance
Lamp intensity, contact time, and general water quality are important determinants of the efficiency of a UV system. The lamp intensity is defined as the dosage of light energy (millijoule, mJ; milliwatt, mW; or microwatt, μW) delivered per unit area of flowing water. The UV treatment systems certified under “NSF standard 55” have minimum safe dosage of 40 mJ/cm2, or otherwise stated as 40 mW/cm2 or 40,000 μW/cm2.
Regardless of the quality of the equipment purchased, it will not perform satisfactorily unless maintained in accordance with the manufacturer’s recommendations for maintenance, cleaning, and part replacement. Keep a record of water test results, equipment maintenance and repairs.
UV lights do not burn out but gradually lose effectiveness with use. The UV lamp should be cleaned on a regular basis and replaced when needed. It is common for a new lamp to lose 20% of its intensity within the first100 hours of operation, although that level is maintained for the next several thousand hours. In general, all UV lamps need to be replaced every 9,000 hours (approximately one year) of continuous lit-up usage or as suggested by the manufacturer. Units equipped with properly calibrated UV emission detectors alert the owner when the unit needs cleaning or the light source is failing–an important feature to ensure safe water supply. A detector that emits a sound or shuts off the water flow is preferable to one with a warning light only.
Color, turbidity, and organic impurities in the water interfere with the transmission of UV light and may reduce efficiency to unsafe levels. Suspended solid particles (turbidity) in the water can shield organisms from exposure to light. The UV light reflects on sediment particles and casts a shadow, much like the shadow that results
when the sun shines on an opaque object. If microorganisms are in this shadow, UV light cannot kill them. To facilitate exposure between the bacteria and light, a sediment filter should free the untreated water entering the unit from turbidity as much as possible.
In addition, iron, manganese, and hardness may reduce the efficiency of the UV unit to effectively treat the water. Hard water salts of calcium and magnesium may coat the quartz sleeve with scale (a whitish deposit of hardness) and reduce the actual output of UV light. Iron and manganese can impart color to the water and reduce the UV light output, alone or together with hard water salts. Frequent cleaning or addition of a water softener to the system may be needed if the water is hard. Commercial products are available for rinsing the unit to remove any film on the light source. Overnight cleaning with a solution of 0.15% sodium hydrosulfite or citric acid effectively removes such films. Some units have wipers to aid the cleaning process.
UV devices are often combined with other technologies such as particle filters, carbon filters, ion exchange units, and reverse osmosis systems to remove particles prior to UV disinfection. Following reverse osmosis, water softening, or filtration, UV is often the last device in the “treatment train,” a series of treatment devices.
UV systems are designed for continuous operation and should be shut down only if treatment is not needed for several days. The lamp needs a few minutes to warm up before the system is used again following a shutdown. In addition, the plumbing system of the house should be thoroughly flushed following a period of no use. Whenever the system is serviced, the entire plumbing system should be disinfected prior to relying on the UV system for disinfection.
Before using a UV system for the first time, disinfect the entire plumbing system with chlorine (See section1.0). The treated water should be tested for coliform bacteria after installation and on a monthly basis after the first six months of use. If bacteria are present in the treated water, the lamp intensity should be checked and the entire plumbing system should be shock chlorinated.
What to look for in an ultraviolet treatment system
Before purchasing a UV treatment system, pay attention to the following information, which the manufacturer should provide:
-
Check whether the product is certified “NSF Standard 55.”
-
Check the manufacturer’s specifications on the dosage. The dosage is the minimum amount of UV energy needed to destroy the pathogens in water. A minimum of 40 mW/cm2 is required.
-
Expect to change your UV lamps once a year and ensure that the manufacturer has spare lamps on hand prior to your purchase.
-
If the water is hard, scaling on the quartz sleeve of the lamp casing will affect the dosage and ultimately the effectiveness of your unit. Ask the manufacturer if its system comes with a valve that automatically purges the system in order to prevent limescale buildup. As an alternative, installing a water softener may be required.
Ozonation
Ozone is a gas that occurs naturally in our atmosphere. By disinfecting water through ozonation, electrically generated ozone kills bacteria and certain other pathogens. Ozone generators are relatively expensive to install. Ozonation does not have any residual disinfection effect in the water, unlike chlorination.
Effective against: pathogenic (disease-causing) organisms, including bacteria and viruses, most amoebic cysts, and aromatic organic compounds (also known as phenols), some color, taste and odor problems, iron and manganese, sulfur, and turbidity.
Not effective against: large cysts and some other large organisms (worms), inorganic chemicals, and heavy metals.
System cost: $250 for a point-of-use (POU) system, $2000 for a point-of-entry (POE) system
How does ozonation treatment work?
Ozone is a strong oxidizing agent, like chlorine, and it kills disease-causing bacteria, viruses, and most amoebic cysts in water in a similar way. Ozone may not kill large cysts and some other large organisms like worms, so these should be eliminated by filtering or other procedures prior to ozone treatment. In combination with an activated carbon or mechanical filter, ozonation oxidizes and precipitates out iron, sulfur, and manganese. Ozone is also effective in eliminating or controlling color, taste, and odor problems. Typically, an ozone dose ranging from 1 to 2 milligrams per liter is sufficient to kill most bacteria and control tastes and odors.
Ozone treatment units are installed as a POU or POE treatment system. Raw water enters one opening and treated water emerges from another. Inside the treatment unit, ozone is produced by an electrical corona discharge or ultraviolet irradiation of dry air or oxygen.
The solubility of ozone in the untreated water also affects treatment. The lower the water temperature, the more ozone is dissolved in the water. Ozone works over a wide pH range, but effectiveness is higher at a pH slightly above 7. The ozone demand is related to the level of contamination in the water, because other substances in the untreated water also react with ozone, using up part of the ozone, leaving less ozone available to treat the targeted contaminants. For proper disinfection, the water to be treated must have negligible color and turbidity levels. An ozone treatment system can also be very energy consumptive.
Advantages and limitations
In contrast to chlorination, the major advantages of ozonation treatment are:
-
It does not add any taste or odor in the water
-
It is effective as a disinfectant over a wide pH and temperature range
-
It generally requires a shorter contact time for bacteria and viruses.
However, deactivation of Giardia and Cryptosporidium requires a longer contact time.
A major limitation of ozonation is that it can produce harmful byproducts in drinking water. For example, if bromide is present in the raw water, ozone reacts with it to form bromate, shown to cause cancer in rats. The EPA has set a drinking water standard for bromate in water at 0.010 milligrams per liter.
Maintenance
Most ozone systems do not require extensive maintenance. Some ozone generation units use an air-drying material to remove the humidity from the air. As routine maintenance, air-drying materials need to be replaced periodically.
In addition, the entire system should be routinely inspected by a water treatment specialist for any ozone leaks. Some ozone systems have monitoring devices that alert the owner to a malfunction or failure by shutting down the entire system, sounding an alarm, or activating a warning light.
Always follow the manufacturer’s instructions for maintenance, cleaning, and part replacement. Regardless of the quality of the equipment purchased, it will not perform satisfactorily unless maintained in accordance with the manufacturer’s recommendations. Keep a logbook to record water test results, equipment maintenance, and repairs.
Other considerations
Following installation, test both the raw water (prior to treatment) and the treated water to ensure it is working properly and removing the targeted contaminants. Periodically testing both the raw and treated water should be continued (quarterly or semiannually) to monitor the system performance. Such testing will also help you determine whether maintenance or replacement of components may be necessary.
Ozone is a toxic gas like chlorine, and ozone generators may leak and could create an ozone hazard within your home, causing illness. Not much is known about the chronic health effects of ozone. Health effects resulting from exposure to 0.1 to 1 mg/L ozone include headache, dry throat, and irritation and burning of the eyes. Ozone can corrode some pipes and fixtures, so it is better to have all surfaces coming in contact with ozone made of ozone- resistant materials, such as stainless steel or Teflon.
Because ozone is very unstable, the greatest drawback with ozone treatment is that it does not produce a reliable residual. With ozone treatment, disinfection occurs primarily within the unit at the point of contact between the ozone and the water. The disinfection effect does not sustain beyond the treatment unit. In contrast, chlorination treatment maintains residual chlorine in the water and continues the disinfection process for some time. Active residual time for ozonation is only several minutes, whereas the active residual time for chlorine is measured in hours. Ozone equipment is one of the most expensive water treatment technologies, and chlorination may still be desirable because of the low residual time for ozone.
Most ozonation units require a storage tank for treated water. Bacteria and microorganisms can grow in the storage tank and in the water distribution system that may require disinfection by flushing and super chlorination. Some systems store treated water in a contact chamber to ensure that water is continuously treated until used.
Ozone treatment systems frequently have pretreatment and post-treatment devices. Filtration may be required as pretreatment to remove excessive turbidity or suspended solids that may shield microorganisms from disinfection or foul the ozone device. Filtration may be necessary post-treatment to remove any oxidized particles.
Most people can smell ozone at a concentration of 0.01 mg/L (well below the level for general comfort) and breathing traces of ozone for a few minutes is of little health concern. To prevent ozone from entering the home, residual ozone should be vented to the outside of the home.
In addition, combustible materials such as gasoline, oil, or grease should not be stored near the ozone system, asozone may cause fire if it is exposed to them.
What to look for when buying an ozonation system
Test your water to determine its contaminants before purchasing an ozonation water treatment device. This will help you determine whether ozone is an appropriate disinfection method for your situation. You should inquire about the following before purchasing an ozone system:
-
Has the treatment system been tested and certified by a third party (e.g., National Sanitation Foundation,NSF) to ensure that it meets manufacturer’s claims?
-
Are there any special installation requirements that may add to the equipment cost, for instance, changes to your household plumbing?
Pasteurization
Water contaminated with microorganisms (bacteria, protozoa, and viruses) does not need to be boiled to kill the microorganisms and make it safe to drink, it only needs to be brought to a temperature that is sufficient to kill all disease-causing organisms. Such disinfection at a lower temperature is known as water pasteurization. Most waterborne bacteria, protozoa and viruses are sensitive to inactivation at 162 °F (or 72 °C), which is much lower than the boiling temperature point of water 212 °F (or 100 °C). Table 1 below indicates the temperatures at which the most common waterborne pathogens are rapidly killed, thus resulting in at least 99.999% (5 log reduction) of the microbes becoming inactivated in five minutes at the given temperature.
Table 1. Waterborne microorganisms and their rapid killing temperature.
| Microbe | Rapid Killing Temperature |
|---|---|
| Worms, Protozoa, (Giardia, Crptosporidium, Entamoeba) cysts | 55 oC (131 oF) |
| Bacteria (Vibrio cholerae, E. coli, Shigelia, Salmonella, etc. and Rotavirus | 60 oC (140 oF) |
| Hepatitis A virus | 65 oC (149 oF) |
Pasteurization can be accomplished in two different ways:
-
Flash pasteurization uses high temperature of 162 °F (or 72 °C) for a short time (15 seconds)
-
Low-temperature pasteurization uses lower temperature of 145 °F (or 63 °C) for a longer time (10 minutes)
Pasteurization results in a great deal of energy savings compared to boiling because the pasteurization temperature is much lower than the boiling temperature. Pasteurization does not leave behind a residual product that continues to disinfect beyond the immediate treatment period.
Distillation
Distillation is one of the oldest water treatment processes. Besides removal of various dissolved and suspended materials, distillation is another water disinfection option. Distillation units boil water, making steam that is condensed and collected as purified water. Generally, distillation is used to supply water only for drinking or special uses.
Effective against: Bacteria, viruses, parasites, heavy metals, dissolved salts such as chloride, fluoride, nitrate,sulfate, and carbonate of sodium, sodium, potassium and magnesium, other dissolved and suspended solids, and some toxic organic chemicals
Not effective against: Most volatile and semivolatile organic compounds (VOCs)
System cost: $250 (counter-top) to over $1450 (larger unit)
How does a distillation unit work?
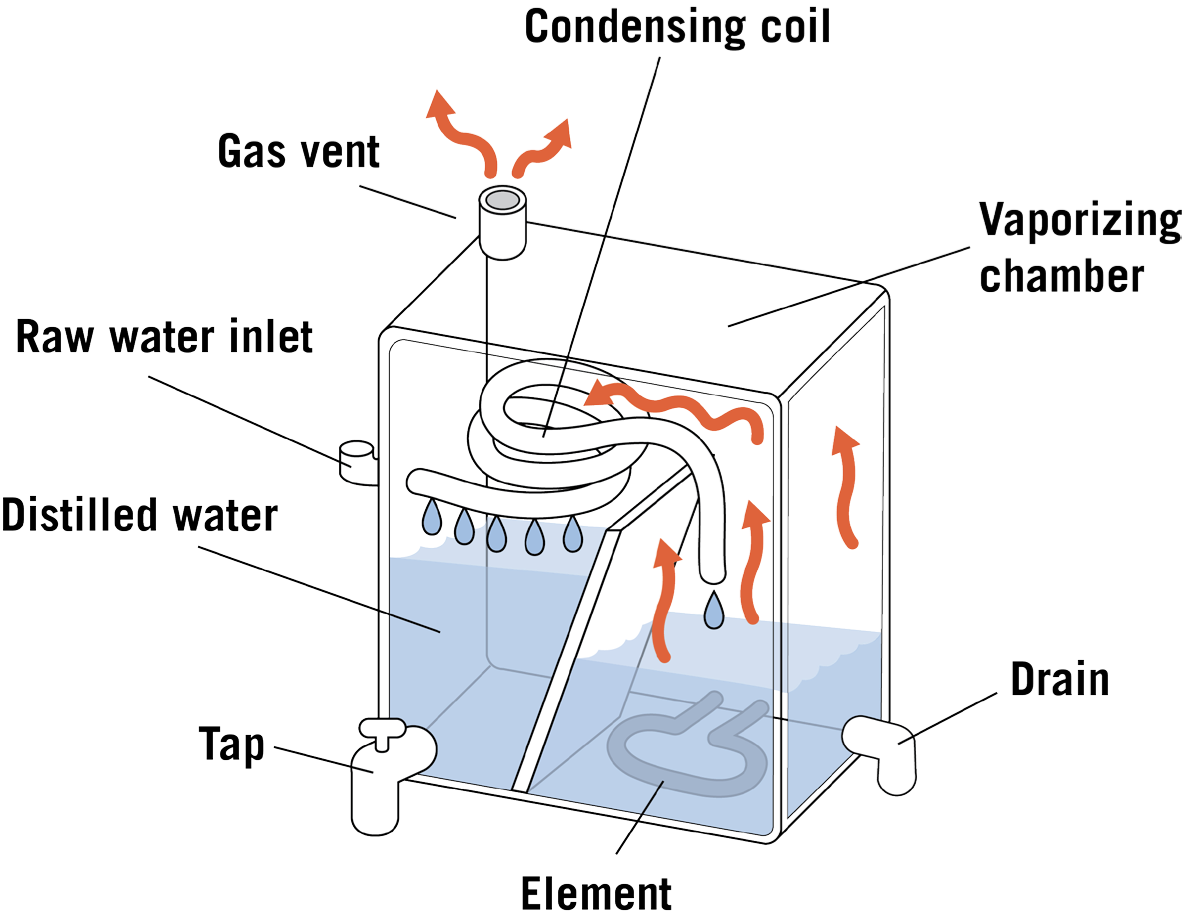
Tap water in a boiling tank (often made of stainless steel) is heated to boiling in a tank. The steam produced enters condensing coils, where it is cooled and condensed back to water. The distilled water goes into a storage container or is piped to a special faucet. The Diagram of a distillation unit is given in Figure 2.
Bacteria are killed during boiling. Storage containers can be glass, metal, or plastic. Each type is satisfactory when cared for as the manufacturer directs. Dissolved minerals and other dissolved and suspended substances are left behind when the steam enters the condensing unit. Distilled water is considered to be relatively pure water. Unevaporated contaminants are left behind and periodically flushed to the septic or sewer system.
Advantages and limitations
The principal advantage of distillation is that this single treatment ensures that treated water is almost pure water and relatively free of many contaminants. Distillation has a special advantage for use on water supplies that have not been chlorinated because disinfection occurs during boiling. Distilled water is suitable for wet batteries and other household equipment requiring mineral-free water. Production of heat from a distiller may be beneficial in the winter but it’s a disadvantage in the summer.
However, distillation suffers from the following limitations:
-
In some distillers, organic contaminants whose boiling point is less than that of water (for example, some pesticides and volatile solvents) are vaporized and carried into the condensation chamber
and will be condensed with the distilled water, which means they remain in the treated water. Chloroform, phenol, and trichloroethylene have been found in distilled water. Others have a volatile gas vent that releases these vaporized contaminants to the atmosphere. -
Distiller tanks, if not properly used and maintained, can also become notorious breeders of bacteria because of the presence of warm water.
-
While removing contaminants, distillers also remove beneficial minerals and make the water taste flat or bland.
-
Distilled waters are generally more acidic than the EPA-recommended pH range of 6.5 to 8.5 for drinking water.
-
The cost of producing distilled water depends on the size of the appliance and the local electric rate. The power consumption of distillation units varies from 3 to 5 kilowatt-hours of electricity per gallon of distilled water produced. In general, the electrical costs of distilled water can be high.
-
The distillation process is very slow. Daily capacity usually ranges from three to twelve gallons.
-
Approximately five gallons of tap water are required to produce one gallon of distilled water, making water waste high.
Distillation equipment
Because distillation units produce a small amount of treated water, distillers are point-of-use rather than point- of-entry systems. They vary from small, round countertop units that distill less than one quart of water per hour to larger, rectangular carts, which distill about one-half gallon of water per hour. Systems can be manual or automated, partially or fully. Some models can separate volatile organic compounds before distillation.
Automatic features on units include reset switches and timers that make automatic operation possible on some models. These features might be desirable when distilled water is not used continuously.
Production rates vary with the type of condensing system. Air-cooled devices typically require two gallons of untreated water for each gallon of treated water. Water-cooled units may require five to 15 gallons of untreated water for each gallon of treated water.
A continuously operating, offline distiller for home use should have a storage tank to hold treated water until itis needed. The storage tank capacity may provide an estimate of a distillation unit’s capacity. Most tanks drain by gravity, but some may have a pump to deliver treated water to other locations.
Maintenance
Regardless of the quality of the equipment purchased, it will not perform satisfactorily unless managed in accordance with the manufacturer’s recommendations for maintenance, cleaning, and part replacement. Keep a logbook to record water test results, equipment maintenance and repairs. The following maintenance activities are considered especially important:
-
After installation, check the boiler chamber and heating element in continuously operating units on a weekly basis for scale accumulation. Check countertop units after each distillation cycle. Develop a regular maintenance schedule based on these initial observations.
-
Any water that remains in the distillation unit is concentrated with minerals and metals, so dispose of it carefully. This will help prevent solids from precipitating and may eliminate some solids that have already precipitated.
-
Cleaning frequency will depend on untreated water quality (especially water hardness) and the amount of water being used. In some cases, the mineral deposits can be dissolved with pure water. In other cases, the mineral built-up needs to be dissolved by dilute acid cleaners, such as lemon juice or white vinegar, or a manufacturer’s cleaner in a heated condition. It is important to keep mineral buildup on the heating element to a minimum, as it reduces heat transfer and results in higher energy costs.
-
Mineral buildup may also necessitate periodically replacing the heating element, although an individual element should last approximately three years.
Other considerations
Distillation units require frequent cleaning and may be difficult to keep clean. Frequent maintenance requirements and substantial electricity consumption should be major considerations when purchasing a distiller. Power usage can be decreased in units with automatic shut-off devices.
After installation, retest both the raw water (prior to treatment) and the treated water to ensure that it is working properly and removing the contaminants. You should continue to test the quality of both the raw and treated water annually or more frequently (quarterly or semiannually) if high levels of contaminants are present in
the raw water. Frequent testing will also help you determine how well your treatment system is working and whether maintenance or replacement of components may be necessary.
What to look for when buying a distillation system
Before purchasing, have your water tested to determine the contaminants present. Consider how much water you need, how contaminated your water supply is, costs, and alternatives like bottled water to determine if distillation is an effective treatment method for your situation. In addition, consider the following:
-
Capacity of the boiling tank
-
Type and size of the water-storage container
-
Rate at which distilled water is produced
-
Presence of automatic features
-
Location of unit for convenience of use and ease of maintenance
-
Wattage rating (650 to 1,500-plus watts)
-
Batch or continuous process mode of operation
-
Testing and certification by a third party (e.g., National Sanitation Foundation) to ensure manufacturer's claims
-
Whether water distillers (other than countertop units) have a drain opening to remove contaminated water
-
Whether there are any special installation requirements that may add to the equipment cost, such as changes to your household plumbing
Disinfection equipment
Before purchasing a water treatment device, have your water tested to determine the contaminants present, whether biological, organic or inorganic. For water testing information including sample collection and submission, visit:
Water testing helps you determine whether the treatment system under consideration is an effective treatment method for your situation. It is also important to get answers to a number of pertinent questions from the water treatment company. For a set of such questions, see section 4.6 in University of Georgia Extension Bulletin 939, Water Quality and Common Treatments for Private Drinking Water Systems.
References
Bergsrud, F., Seelig, B., & Derickson, R. (1992). Treatment Systems for Household Water Supplies: Chlorination, AE-1046. North Dakota State University.
Connecticut Department of Public Health, Environmental Health Section, Private Well Program (2009). Private Drinking Water in Connecticut: Ozone Treatment of Private Drinking Water Systems, Publication No. 17.
Dvorak, B.I., & Skipton, S.O. (2013). Drinking Water Treatment: Distillation, G1493. Institute of Agriculture andNatural Resources at the University of Nebraska-Lincoln.
Dvorak, B.I., & Skipton, S.O. (2016). Drinking Water Treatment: Continuous Chlorination, G1496. Institute ofAgriculture and Natural Resources, University of Nebraska-Lincoln.
Herman, G.M., & Jennings, G.D. (1996). Home Drinking Water Treatment Systems. North Carolina CooperativeExtension Service, Publication Number: HE-419.
Ozone Treatment of Drinking Water Supplies, Healthy Drinking Waters for Massachusetts, University of Massachusetts Extension, 2007.
Oram, B. Drinking Water Treatment with UV Irradiation. Water Research Center. https://www.knowyourh2o.com/indoor-4/uv-disinfection
Parrotta, M.J., & Bekdash, R. (1998). UV disinfection of small groundwater supplies. Journal AWWA. 90(2):71-81
Ultraviolet Light Disinfection of Drinking Water: An Alternative to Chlorination. National Water Quality Program.
U.S. Environmental Protection Agency, Office of Groundwater and Drinking Water (1996). Ultraviolet LightDisinfection Technology in Drinking Water Application: An Overview, EPA-811-R-96-002, National Service Center for Environmental Publications (NSCEP).
Wagenet, L., Mancl, K., & Sailus, M. (1995). Home Water Treatment. Northeast Regional Agricultural Engineering Service, Cooperative Extension. NRAES-48.
Status and Revision History
Published on Apr 11, 2018
Published with Full Review on Jun 09, 2023


























































