Watermelon (Citrullus lanatus) is an economically important vegetable commodity in Georgia. In 2015, the watermelon crop was valued at more than $124 million and was grown on nearly 20,000 acres.1 Typically, Georgia is ranked second or third in watermelon production nationally. Nearly all production is seedless, with seeded melons being used as pollenizers.2 Watermelon in the Southeast United States are grown either on bare ground or in plastic mulch (Figure 1).
Watermelon production faces many challenges in Georgia. The warm, humid climate favors numerous foliar diseases. Because of the long history of watermelon production in Georgia, soilborne diseases such as fusarium wilt also present challenges to growers. Fusarium wilt of watermelon is caused by the fungal pathogen Fusarium oxyporum f. sp. niveum, or FON (Figure 2).
FON was first described in Georgia and South Carolina in 1890 by Erwin F. Smith, a renowned U.S. Department of Agriculture plant pathologist. Smith’s research includes the earliest work on fusarium wilt of watermelon and includes descriptions of the pathogen, inoculation, and host specificity studies (Figure 3).3, 4
Since Smith’s discovery in 1894, FON has been the most important disease of watermelon in the world.5 The disease has far-reaching implications due to its ability to overwhelm management strategies.


Yield losses from FON are increasing around the world.6 Losses can be attributed to several limiting factors, such as pathogen biology, survival mechanisms, and the evolution of pathological races. Biological characteristics, like long-term spore survival, have complicated management strategies since the pathogen’s discovery. The hard- resting spores of FON, called “chlamydospores,” can live in the soil without a host plant for 15 years or more, making short-term crop rotations ineffective.7 Implications of this disease can be significant in areas such as the Southeast U.S., where it is hard to follow long-term crop rotation recommendations because of limited availability of irrigated land. Years of monocropping aided in the selection for new races of FON, such as the aggressive race 2, and more recently, race 3. Race 2 has now been confirmed in eight states, including the state of Georgia.6 There are numerous commercially available seeded and seedless varieties with resistance to FON race 1. However, many growers have race 2 and possibly race 3 in their fields. While there is some level of resistance to race 2 in some non-harvestable pollenizer (seeded melon) varieties, there are currently no commercially available seedless varieties with resistance to race 2 of FON. The evolution of race 3 has further complicated cultivar-resistance strategies. Further analysis of distribution and progression of race 3 is needed to determine the scope of race 3 development.
Because resistance to FON is limited in seedless melons, we must rely on other management strategies to successfully combat this disease. Historically, the fumigant methyl bromide was widely used for control of many soilborne pathogens. However, because methyl bromide has been linked to damage to the ozone layer, its use has been phased out in accordance to the Montreal Protocol.8 The discontinuance of methyl bromide left a void in chemical management strategies against soilborne organisms, including. New approaches to managing FON will be multifaceted, incorporating fumigants, fungicides, plastic mulches, planting dates, and varieties.

Symptoms
Many factors can influence the symptom development of fusarium wilt. The amount of inoculum in the soil, environmental conditions, nutrients, and susceptibility of the host may impact the severity of the disease.5 Fusarium wilt is the most severe in sandy, acidic soils at temperatures between 25–27 °C. Initial onset of Fusarium wilt is characterized by foliage transition from healthy green tissue to dim gray/green coloration. Foliage discoloration is followed by the loss of turgor pressure and general chlorosis of tissue. Wilting occurs soon after. Wilting is often observed in a single runner at first, progressing to the rest of the plant soon after. Initial recovery from wilting may occur during the evening hours, but it eventually becomes permanent (Figure 4).


Older parts of the plant including the stem may have brown necrotic lesions that are close in proximity to the soil line or the crown. The plants can get infected early; however, symptoms can be readily seen after fruit set. The primary diagnostic method for fusarium wilt is to section the stem to look for vascular discoloration.5 Unilateral stem necrosis can be seen on older stems that have been longitudinally severed. Under high moisture conditions, white to pink hyphae may be observed on necrotic lesions from the soil line.
FON may also cause stunting, but stunted plants may not always have conspicuous symptoms that are characteristic of FON. Highly susceptible plants may experience complete plant wilt, resulting in death. Severe wilt can cause death in as little as 10 days after infection.9 Generally, plants in the field start to experience symptoms within three to four weeks of planting. After fruit set, disease severity often increases and plants that appear asymptomatic may start to express symptoms. Symptomatic vines are often clustered together as a result of inoculum distribution in the soil.
Pathogen survival and infection
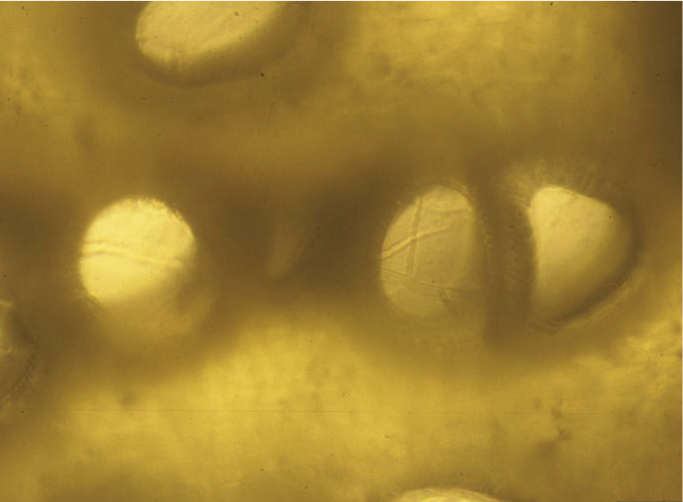
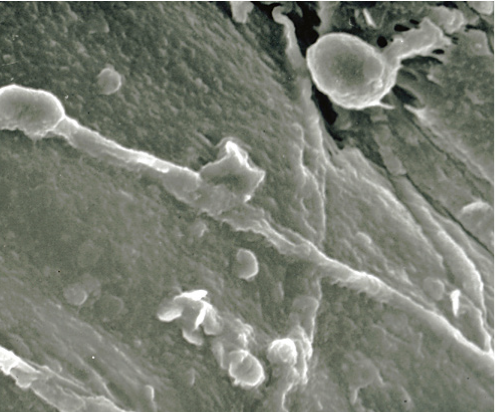
In 1895, one year after the discovery of F. oxysporum f. sp. niveum, E.F Smith determined that FON could survive for long periods in the soil in the absence of a host. Chlamydospores are the primary means of survival in the soil. Chlamydospores can remain viable for 15 years or more.6 Infection occurs when chlamydospores germinate to form hyphae that penetrate the root cortex of the watermelon plant. After the colonization of the cortex, mycelia, which are thread-like, branching hyphae, move into the xylem tissue and produce microconidia, smaller masses of asexual fungal spores (Figures 5 and 6).5
Microconidia are carried down the xylem vessels where they may germinate to create more mycelia and microconidia. Because of this, the fungus spreads quickly throughout the plant, causing a systemic infection. FON-mediated wilt symptoms are the result of a host’s resistance response to this infection.
When FON mycelia enter the xylem, the watermelon plant produces tyloses. Tyloses are projections (or swellings) of the parenchyma tissue that inhibit the flow of water through the xylem, or the vascular tissue through which water and nutrients flow. Tyloses prevent the fungal tissue from spreading throughout the plant. In resistant cultivars, tyloses are formed quickly enough to block further movement of the pathogen. But susceptible plants do not form tyloses quickly, and mycelia and spores are dispersed throughout the plant. Additionally, the pathogen breaks down parenchyma cells that cause gumming of the xylem. Together, tyloses and gumming of the xylem block water transfer and cause the characteristic vascular wilt (Figure 5). The discoloration of vascular tissue is due to the gumming of xylem vessels.
After infection, the pathogen more often resides within the watermelon plant until the plant dies or starts to decay. Upon the death of the plant, mycelia move to the exterior of the stem and begin production of macroconidia. Mycelia and macroconidia are incorporated into the soil, and under stressful environmental conditions, chlamydospores are formed from the aforementioned pathogen structures. Because chlamydospores are the primary means of survival of fusarium wilt, the spread of the disease is achieved mostly by dissemination of chlamydospores. Chlamydospores can be spread throughout the field by mechanical methods such as rainfall, tractor equipment, and operator movement. Fusarium wilt may also spread by seed.10
As far back as 1928, FON was isolated from infected seeds. Since then there have been multiple reports confirming FON’s seedborne nature. The extent of seedborne spread is largely unknown, although infection frequencies are generally less than 5%.7 Other mechanisms of survival include survival on plant debris and colonization of living non-host plants.
Taxonomy
Fusarium species are extremely diverse and among some of the most important plant pathogens in the world. The genus Fusarium causes disease on nearly every economically important plant species.11 Similarly, Fusarium oxysporum species are extremely diverse and cause disease on an array of plant species.7 Over 120 formae speciales have been classified based on their host specificity. E.F. Smith named the special form that infects watermelon “niveum,” after the Latin “niveus,” which means “white” or “snow.” The reason behind this nomenclature is the characteristic white hyphae that may be observed growing from the stem of the infected plant.7
Morphological Characteristics
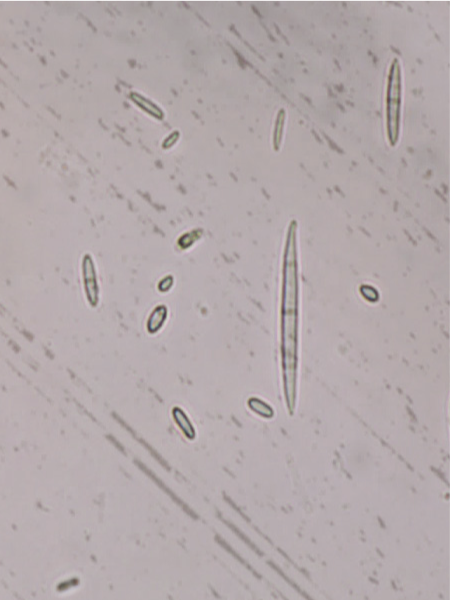
FON reproduces by microconidia and macroconidia. Microconidia are produced on short conidiophores and are small, single-celled, and kidney-shaped (Figure 7). They have the ability to infect host roots but rarely do so because of their short life span.5 Macroconidia are fusiform (boat-shaped) and often have three to five cells (Figure 7). Macroconidia have the ability to form chlamydospores. Chlamydospores may arise from macroconidia or mycelia and are formed under unfavorable environmental conditions
Pathogen Identification
FON is most commonly identified by spore morphology. The different formae speciales of FON are not morphologically discernable, so screening should is necessary for identification past the species level.9 Fungal structures including conidia, conidiophores, and chlamydospores can be identified under a compound microscope.
Pathogenic Races
Races of FON are characterized by their ability to overcome various resistance genes that differ with particular watermelon cultivars.5 Tests are performed on a set of varieties with known resistance levels to identify the race of FON present (Table 1). Four races of FON have been identified: race 0, 1, 2, and 3.7 ‘Sugar Baby’ and ‘Black Diamond’ watermelon cultivars are susceptible to all races of F. oxysporum. ‘Charleston Gray’ and ‘Crimson Sweet’ are resistant to race 0 but susceptible to races 1, 2, and 3. ‘Calhoun Gray’ and ‘Allsweet’ are resistant to races 0 and 1 and susceptible to races 2 and 3. And the PI-296341-FR variety is resistant to all races of FON with the exception of race 3.
Table 1. Watermelon genotypes used to determine races of Fusarium oxysporum f. sp. niveum.
|
Genotype |
Race 0 |
Race 1 |
Race 2 |
Race 3 |
|
‘Sugar Baby’ |
S |
S |
S |
S |
|
‘Black Diamond’ |
||||
|
‘Charleston Gray’ |
R |
S |
S |
S |
|
‘Crimson Sweet’ |
||||
|
‘Calhoun Gray’ |
R |
R |
S |
S |
|
‘Allsweet’ |
||||
|
PI-296341-FR |
R |
R |
R |
S |
Each race has various levels of aggressiveness, with race 0 being the least aggressive and race 3 being most aggressive. Race 1 was first described in South Carolina and Georgia in 1894.3 Almost a century later, race 0 was differentiated from race 1 on the basis of its aggressiveness.13 Race 0 has little economic importance because most commercial watermelon varieties possess the resistance gene for race 0.7 Race 1 is the most commonly found race in the United States and worldwide. Race 2 was initially observed in three states, Texas, Oklahoma, and Florida. Since then race 2 has been disseminated into eight watermelon-producing states, including the first report in Georgia in 2004.14, 15 The newest addition to the race complex, race 3, was first observed in Maryland,16 but it has also been documented by the authors in Georgia.
Management
Due to its long-term survival in the soil and the ability of the pathogen to evolve into new races, controlling fusarium wilt is challenging. Various control methods such as fumigation, planting date, fungicides, and grafting plants have been evaluated with varying degrees of success. Crop rotation is by far the oldest fusarium wilt control method.6 Crop rotation can be a valuable management tool in many cropping situations, but the long-term spore survival of FON complicates traditional rotation strategies. Rotations of five to seven years have been proposed, but even they may not be long enough to effectively eradicate the pathogen, as chlamydospores can survive up to 15 years in the soil.7, 17 Unlike crop rotation, cultivar resistance is economical and can be easily implemented by growers. However, current seedless cultivars only have resistance to races 0 and 1. A pollenizer variety, Sp6, does currently offer resistance to race 2. Additional management strategies including using disease-free seed and transplants are also important to reduce the severity of disease. Avoidance of diseased material should always be the first defense in a successful fusarium wilt control program. Watermelon transplants should be monitored for signs and symptoms of disease. Restricting movement of diseased transplants will prevent FON introduction into new fields and will limit the dissemination of new races.5
Soil solarization is an additional management strategy that uses clear plastic mulch placed over the soil to increase the temperature to levels that are lethal to the pathogen. Research has shown that soil solarization significantly delays the onset of fusarium wilt symptoms.7 Solarization does not provide complete control of fusarium wilt, however—spores may still be present in the soil after treatment.5 The technique is rarely used in the U.S. because it requires long, uninterrupted periods of hot, sunny weather to achieve lethal soil temperatures. Furthermore, it is labor-intensive and cost-prohibitive.
Grafting
Grafting seedless watermelons onto squash or gourd rootstocks that are resistant to FON has gained importance throughout watermelon growing regions around the world (Figure 8).6Research conducted in the Southeast U.S. by Keinath and Hassell (2014) showed an 88% reduction of fusarium wilt incidence in grafted transplants when compared to non-grafted transplants. Grafted transplants have also been reported to produce higher yields than non-grafted varieties. Although analysis of resistant grafts for the control of fusarium wilt has shown favorable results, complications do exist. Resistant grafts are labor-intensive to produce and are expensive.

Fumigation
In 1997, the United States signed on to the Montreal Protocol, which called for the complete phaseout of methyl bromide by 2015.8 For many years, methyl bromide was the gold standard of soil fumigants and controlled a wide variety of soilborne pests.7Fumigation provided the most economical and effective control against fusarium wilt.19 Since methyl bromide has been phased out, research has been focused on identifying an efficacious soil fumigant for fusarium wilt control.6 Alternatives include chloropicrin, methyl iodide, and metam sodium.5, 7 To date, no effective alternative fumigants are available that can provide control as well as methyl bromide.19
Chemical Control

Historically, fumigants have been the primary chemical management practice for the control of fusarium wilt, but interest in fungicides have increased since the phaseout of methyl bromide. Until recently, there have been no conventional soil-applied herbicides to control fusarium wilt.6 In 2013, Bayer CropScience LP’s Research Triangle Park in North Carolina received a supplemental label for Proline (prothioconazole).14 Proline can be applied by either ground or chemigation application equipment, including drip application. It is not labeled for use in water for transplanting, nor is it labeled for use in greenhouses or transplant houses. The label allows drip irrigation of Proline with certain restrictions.14 The capability of applying fungicide through drip irrigation for fusarium wilt control is a new concept and additional research is needed to determine season-long efficacy(Figure 9).6 Controlling fusarium wilt with chemicals is extremely challenging.7 Future management programs must involve multiple tactics, and labeled fungicides will provide an added component that has not been available in the past.
References
-
University of Georgia Department of Agriculture and Environmental Science Center for Agribusiness & Economic Development (2015). 2014 Georgia Farmgate Value Report (K. Wolfe & K. Stubbs, Comps.).
-
U.S. Watermelon Statistics, 1950-2012. (2012). Retrieved from National Agricultural Statistics Service United States Department of Agriculture database.
-
Smith, E.F. (1894). The watermelon disease of the South. Proceedings of the American Association for the Advancement of Science Sec. G.43,289–290.
-
Smith, E.F. (1899). Wilt disease of cotton, watermelon, and cowpea (Neocosmospora nov. gen.) by Erwin F. Smith. In , Bulletin / U.S. Department of Agriculture, Division of Vegetable Physiology and Pathology ; no. 17 Washington : G.P.O.
-
Egel, D.S., & Martyn, R.D. (2007). Fusarium wilt of watermelon and other cucurbits. The Plant Health Instructor. DOI: 10.1094/PHI- I-2007-0122-01. Updated 2013.
-
Everts, K.L., & Himmelstein, J.C. (2015). Fusarium wilt of watermelon: Towards sustainable management of a re-emerging plant disease. Crop Protection, 73, 93-99. doi:10.1016/j.cropro.2015.02.019
-
Martyn, R.D. (2014). Fusarium wilt of watermelon: 120 years of research. Horticultural Reviews, 42, 349-441.
-
Gullino, M.L., Camponogara, A., Gasparrini, G., Rizzo, V., Clini, C., and Garibaldi, A. (2003). Replacing methyl bromide for soil disinfestation:the Italian experience and implications for other countries. Plant Disease, 87(9), 1012-1021.
-
Kleczewski, N.M., and Egel, D.S. (2011). A diagnostic guide for Fusarium wilt of watermelon. Plant Health Progress doi:10.1094/PHP-2011-1129-01-DG.
-
Martyn, R.D., and Vakalounakis, D.J. (2012). Fusarium wilt of greenhouse cucurbits: Melon, watermelon and cucumber. p. 159–174. In: M. Lodivica Gullino, J. Katan, and A. Garbaldi (eds.), Fusarium wilts of greenhouse vegetable and ornamental crops. APS Press, St. Paul, MN.
-
Ma, L.-J., van der Does, H., Borkovitch, K.A., Coleman, J.J., et al. (2010). Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature 464:367–373.
-
Michielse, C.B. and Rep, M. (2009). Pathogen profile update: Fusarium oxysporum. Molecular Plant Pathology, 10, 311–324. doi:10.1111/ j.1364-3703.2009.00538.x
-
Crall, J.M. (1963) Physiologic specialization in Fusarium oxysporum F. sp. niveum. Phytopathology 53, 873 (Abstract).
-
Everts, K.L., Egel, D., Langston, D., and Zhou, X. (2014). Chemical management of Fusarium wilt of watermelon. Crop Protection, 66, 114-119.
-
Bruton, B.D., Fish, W.W., and Langston, D. B. (2008). First report of Fusarium wilt caused by Fusarium oxysporum F. sp. niveum race 2 inGeorgia watermelons. Plant Disease, 92(6), 983. DOI:10.1094/PDIS-92-6-0983B
-
Zhou, X., Everts, K., and Bruton, B. (2010). Race 3, a New and Highly Virulent Race of Fusarium oxysporum F. sp niveum Causing Fusariumwilt in Watermelon. Plant Disease, 94(1), 92-98.
-
Zhou, X.G., and Everts, K.L. (2004). Suppression of Fusarium wilt of watermelon by soil amendment with hairy vetch. Plant Disease. 88, 1357- 1365.
-
Keinath, A.P, and Hassell, R.L. (2014). Control of Fusarium wilt of watermelon by grafting onto bottlegourd or interspecific hybrid squash despite colonization of rootstocks by Fusarium. Plant Disease, 98(2), 255-266. doi: 10.1094/PDIS-01-13-0100-RE
-
Wechter, W.P., Kousik, C.S., McMillan, M.L., Levi, A (2012) Identification of resistance to Fusarium oxysporum f. sp. niveum race 2 in Citrul-lus lanatus var. citroides plant introductions. HortScience 47:334–338.
Status and Revision History
Published on Jan 19, 2018
Published with Full Review on Apr 29, 2024


























































