- Organics (BOD, COD, TOC, O&G)
- BOD (biochemical oxygen demand)
- COD (chemical oxygen demand)
- TOC (total organic carbon)
- O&G (oil and grease)
- Selected References
Since the implementation of the Clean Water Act and subsequent creation of the U.S. Environmental Protection Agency (EPA) in the early 1970s, industrial, institutional, and commercial entities have been required to continually improve the quality of their process wastewater effluent discharges.
At the same time, population and production increases have increased water use, creating a corresponding rise in wastewater quantity. This increased water use and process wastewater generation requires more efficient removal of by-products and pollutants that allows for effluent discharge within established environmental regulatory limits.
The determination of wastewater quality set forth in environmental permits has been established since the 1970s in a series of laboratory tests focused on four major categories:
- Organics - A determination of the concentration of carbon-based (i.e., organic) compounds aimed at establishing the relative “strength” of wastewater [e.g., biochemical oxygen demand (BOD), chemical oxygen demand (COD), total organic carbon (TOC), and oil and grease (O&G)].
- Solids - A measurement of the concentration of particulate solids that can dissolve or suspend in wastewater [e.g., total solids (TS), total suspended solids (TSS), total dissolved solids (TDS), total volatile solids (TVS), and total fixed solids (TFS)].
- Nutrients - A measurement of the concentration of targeted nutrients (e.g., nitrogen and phosphorus) that can contribute to the acceleration of eutrophication (i.e., the natural aging of water bodies).
- Physical Properties and Other Impact Parameters - Analytical tests designed to measure a varied group of constituents directly impact wastewater treatability (e.g., temperature, color, pH, turbidity, odor).
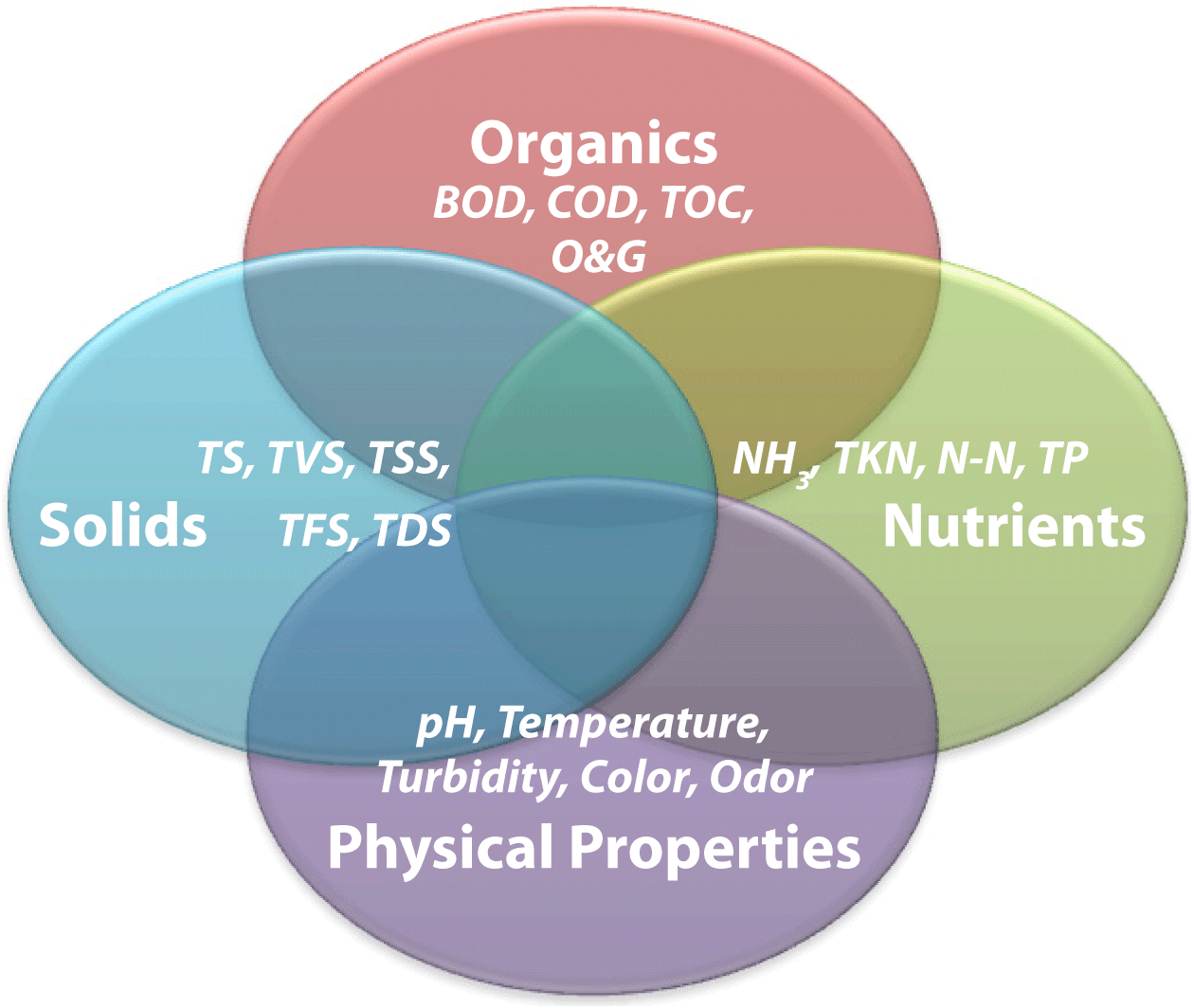 Figure 1. Interaction of wastewater analytical categories and laboratory tests.
Figure 1. Interaction of wastewater analytical categories and laboratory tests.Although wastewater analytical tests are often separated into categories, it is important to understand that these tests are not independent of each other (Figure 1). In other words, a potential contaminant identified by one test in one category can also be identified in another test in a separate category. For example, the organics in a wastewater sample represented by BOD will also be represented in the spectrum of solids, either as suspended (TSS) or dissolved (TDS) particulates. For most people a complete understanding of the standard methods required to accurately complete critical wastewater analytical tests is not necessary. However, a fundamental understanding of the theory behind, and working knowledge of, the basic procedures used for each test and the answers to commonly asked questions about each test can be a valuable tool for anyone involved in generating, monitoring, treating, or discharging process wastewater.
This publication is designed to provide a solid working knowledge of one of the major wastewater analytical test categories: Organics.
Organics (BOD, COD, TOC, O&G)
Analytical tests aimed at establishing the concentration (typically in mg/L or ppm) of organic (i.e., carbon-containing) matter have traditionally been used to determine the relative "strength" of a wastewater sample. Today there are four common laboratory tests used to determine the gross amount of organic matter (i.e., concentrations > 1.0 mg/L) in wastewater:
- BOD (biochemical oxygen demand)
- COD (chemical oxygen demand)
- TOC (total organic carbon)
- O&G (oil and grease)
Is My Wastewater "High-Strength"?
Wastewater generated by commercial, industrial and institutional facilities is typically referred to as "high-strength" compared to typical household wastewater. Table 1 shows the typical concentrations (mg/L) of organics found in untreated domestic wastewater. This table can be used to understand how non-sanitary process wastewater compares to typical domestic wastewater.
| Table 1. Typical concentrations of organics in untreated domestic wastewater. | ||||
| Constituents | Unit | Typical Concentration | ||
| Low | Medium | High | ||
| BOD (biochemical oxygen demand) | mg/L | 110 | 190 | 350 |
| COD (chemical oxygen demand) | mg/L | 250 | 430 | 800 |
| TOC (total organic carbon) | mg/L | 80 | 140 | 260 |
| O&G (oil and grease) | mg/L | 50 | 90 | 100 |
| Adapted from Metcalf & Eddy, Inc., 2003 | ||||

Wastewater Analytics Acronyms:
Organics:
- BOD - biochemical oxygen demand
- COD - chemical oxygen demand
- TOC - total organic carbon
- O&G - oil and grease
Solids:
- TS - total solids
- TSS - total suspended solids
- TDS - total dissolved solids
- TVS - total volatile solids
- TFS - total fixed solids
Nutrients:
- NH3 - ammonia
- TKN - total Kjeldahl nitrogen
- N-N - nitrite/nitrate
- TP - total phosphorus
BOD (biochemical oxygen demand)
- BOD is the traditional, most widely used test to establish concentration of organic matter in wastewater samples (i.e., relative strength).
- BOD is based on the principle that if sufficient oxygen is available, aerobic biological decomposition (i.e., stabilization of organic waste) by microorganisms will continue until all waste is consumed.
- The BOD test is also known as "BOD5" since it is based on the accurate measure of DO (dissolved oxygen) at the beginning and end of a five-day period in which the sample is held in dark, incubated conditions (i.e., 20°C or 68°F).
- The change in DO concentration over five days represents the "oxygen demand" for respiration by the aerobic biological microorganisms in the sample.
- The five-day completion window is an inherent disadvantage of the test because wastewater treatment system personnel cannot use it to make real-time operational adjustments.
- An extended UBOD (ultimate BOD) test that measures oxygen consumption after 60 days or more is sometimes required in wastewater permits.
BOD Test Procedures
- To ensure proper biological activity during the BOD test, a wastewater sample:
- Must be free of chlorine. If chlorine is present in the sample, a dechlorination chemical (e.g, sodium sulfite) must be added prior to testing.
- Needs to be in the pH range of 6.5-7.5 S.U. If the sample is outside this range, then acid or base must be added as needed.
- Needs to have an existing adequate microbiological population. If the microbial population is inadequate or unknown, a "seed" solution of bacteria is added along with an essential nutrient buffer solution that ensures bacteria population vitality.
- Specialized 300 ml BOD bottles designed to allow full filling with no air space and provide an airtight seal are used. The bottles are filled with the sample to be tested or dilution (distilled or deionized) water and various amounts of the wastewater sample are added to reflect different dilutions. At least one bottle is filled only with dilution water as a control or "blank."
- A DO meter is used to measure the initial dissolved oxygen concentration (mg/L) in each bottle, which should be a least 8.0 mg/L. Each bottle in then placed into a dark incubator at 20 °C for five days.
- After five days (± 3 hours) the DO meter is used again to measure a final dissolved oxygen concentration (mg/L), which ideally will be a reduction of at least 4.0 mg/L.
- The final DO reading is then subtracted from the initial DO reading and the result is the BOD concentration (mg/L). If the wastewater sample required dilution, the BOD concentration reading is multiplied by the dilution factor.
| APHA Standard Methods for BOD Measurement | |
| 5210 B. | 5-Day BOD Test 1, 2 |
| 5210 C. | Ultimate BOD Test |
| 5210 D. | Respirometric Method |
| 1 5210 B. is the only EPA approved BOD method 2 Most popular method |
|
What is DO (dissolved oxygen)?
- As the name implies, a DO test measures the concentration of oxygen dissolved in a water or wastewater sample.
- DO measurement most often takes place using an electronic meter fitted with a specialized DO probe.
- The concentration of DO in a water sample is significantly influenced by:
- Temperature: As water temperature increases, DO decreases (i.e., as water gets warmer, it holds less oxygen) (Table 2).
- Salinity: As water salinity increases, DO decreases (i.e., as water gets saltier, it holds less oxygen).
- Atmospheric Pressure: As pressure increases, DO also increases (i.e., water holds less oxygen as you increase altitude).
| Table 2. Effect of temperature on oxygen saturation at 1 atmospheric pressure (i.e., sea level)* | ||
| Temperature |
Concentration (mg/L) |
|
| °C | °F | |
| 0 | 32 | 14.6 |
| 5 | 41 | 13.1 |
| 10 | 50 | 11.3 |
| 15 | 59 | 10.1 |
| 20 | 68 | 9.1** |
| 25 | 77 | 8.2 |
| * Adapted from Metcalf & Eddy, 2003 ** BOD test method temperature |
||
How can my wastewater have a BOD of 1,500 mg/L when clean water at 68 °F can only contain 9.1 mg/L of DO (Table 2)?
The answer is serial dilution—a procedure that allows for the stepwise reduction in concentration (usually 10-fold) of full strength wastewater in DI (deionized) water, as illustrated in Figure 2 below.
After dilution, the resulting difference between the initial and final DO reading simply has to be multiplied by the dilution factor to determine the final BOD result. For example: 1.0 ml of a full strength wastewater sample added to 9.0 ml of DI water results in a 0.1 dilution of the wastewater. The DO concentration (mg/L) reduction must then be multiplied by 10 to determine the final BOD concentration.
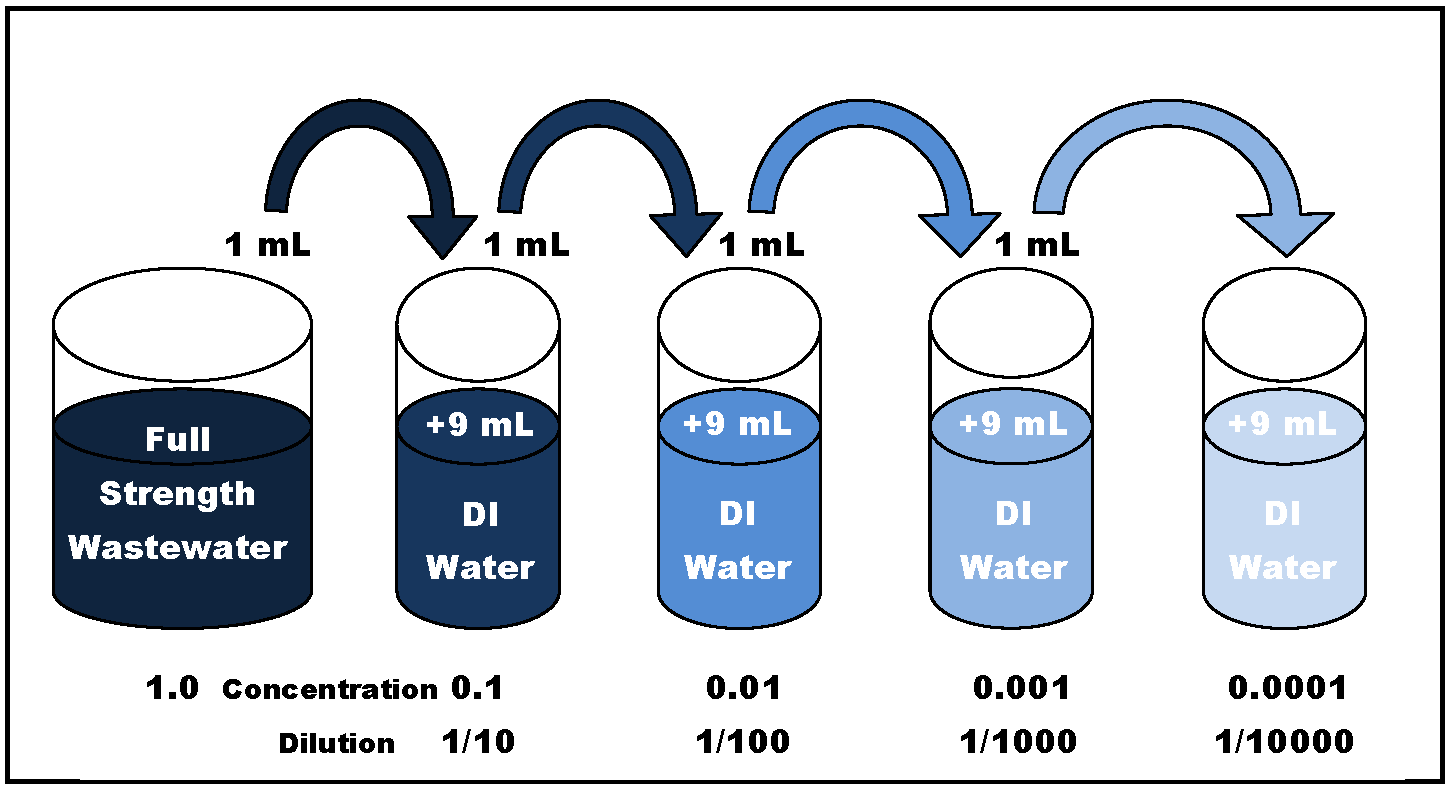
Figure 2. Logarithmic (10-Fold) Serial Dilution of Wastewater
My lab reports lists my results in “ppm.” What does that mean?
Most wastewater test results will be reported in either milligrams per liter (mg/L) or parts per million (ppm). The good news is these two units are equal and thus are interchangeable! However, make sure you always note the units reported. Some wastewater parameters (e.g., heavy metals) are often reported in smaller units such as micrograms per liter (µm/L) or parts per billion (ppb).
COD (chemical oxygen demand)
- COD is the most popular alternative test to BOD for establishing the concentration of organic matter in wastewater samples.
- The COD test only takes a few hours to complete, giving it a major advantage over the 5-day BOD test. Wastewater treatment system personnel can use COD as an almost real-time operational adjustment parameter.
- COD can test wastewater that is too toxic for the BOD test.
- The COD test should be considered an independent measure of the organic matter in a wastewater sample rather than a substitute for the BOD test.
- The COD test uses a chemical (potassium dichromate in a 50% sulfuric acid solution) that “oxidizes” both organic (predominate) and inorganic substances in a wastewater sample, which results in a higher COD concentration than BOD concentration for the same wastewater sample since only organic compounds are consumed during BOD testing.
- The most popular current testing method for COD involves using sealed and heated (i.e., closed reflux) low-range (3-150 ppm) or high-range (20-1500 ppm) pre-prepared vials that change color from orange to green based on the amount of oxidation and that are read using a laboratory colorimeter
COD Test Procedures
- Prior to completing the COD test, a series of known standards are prepared using KHP (potassium hydrogen phthalate). Most wastewater samples will fall in the high range, so standards of 100, 250, 500 and 1000 mg/L are typically prepared. COD standards can also be purchased.
- A COD reactor/heating (150°C) block and a colorimeter are turned on so that both instruments are allowed to stabilize.
- Pre-prepared low-range (3-50 ppm) or high-range (20-1500 ppm) vials are selected for the COD test based on expected results. Both ranges can be used if expected results are unknown.
- One vial is marked as a “blank,” and three or four vials are marked with known standard levels. Two vials are then marked for the wastewater sample to make a duplicate run. Note: If multiple wastewater samples are being run, at least 10% of samples are duplicated.
- 2 ml of liquid are added to each vial. In the case of the “blank,” 2 ml of DI water are added. 2 ml of each standard are added to the corresponding vials. If the wastewater sample is tested at full strength, then 2 ml is added to the corresponding vial. If dilution is required, then serial dilutions are performed and 2 ml of the diluted sample are added to the corresponding vial.
- Each vial is mixed well and placed into the reactor block for two hours. After two hours, the vials are removed from the block to a cooling rack for about 15 minutes.
- The colorimeter is set and calibrated per the specific instructions for that unit (i.e., proper wavelength, blank and standards) and each vial is placed in the unit and the COD concentration read.
- If the sample was diluted, the corresponding multiplication is made.
| APHA Standard Methods for COD Measurement | |
| 5220 B. | Open Reflux Method |
| 5220 C. | Closed Reflux, Titrimetric Method 1 |
| 5220 D. | Closed Reflux, Colorimetric Method 1, 2 |
| 1 EPA Approved Method 2 Most popular method |
|
Can I use my COD results to predict my BOD?
YES. Although COD should be considered an independent test from BOD, and will generate a higher concentration reading than BOD for a particular wastewater sample, it is generally accepted that COD and BOD share an empirical relationship. Extensive observation of the COD and BOD levels on the same wastewater has shown that the COD to BOD ratio of a particular wastewater will remain constant over time.
For example, food processing wastewater will generally have a COD:BOD ratio of ~2:1, while textile wastewater that can contain dyes will often have a much higher COD:BOD ratio of ~5:1.
To establish the COD:BOD ratio for your wastewater, simply have both COD and BOD run on several wastewater samples. Divide the COD concentration by the BOD concentration for each sample and average the results. For example, below is the COD:BOD ratio developed using three wastewater samples from a food processor:
The important point is that once you have established an average COD:BOD ratio for your wastewater stream, then the relatively simple and quick COD test can be used to predict BOD with relative reliability.
| Sample 1: | COD = 2,150 mg/L | BOD = 1,100 mg/L |
| COD ÷ BOD = 2,150 ÷ 1,100 = 1.95 | ||
| Sample 2: | COD = 1,990 mg/L | BOD = 1,050 mg/L |
| COD ÷ BOD = 1,990 ÷ 1,050 = 1.89 | ||
| Sample 3: | COD = 1,850 mg/L | BOD = 997 mg/L |
| COD ÷ BOD = 1,850 ÷ 997 = 1.86 | ||
| (1.95 + 1.89 + 1.86) ÷ 3 = 1.9 COD:BOD Ratio = 1.9:1 |
||
| Note: Three samples are used for this example, but 3 samples are too few to calculate an accurate ratio. It is recommended that a minimum of 10 samples be used to develop an initial ratio and that the ratio is consistently updated based on additional periodic sample results. | ||
WARNING! COD…Hazardous Waste
Along with the potassium dichromate in 50% sulfuric acid solution, pre-prepared COD vials also contain silver sulfate as a catalyst and mercuric sulfate to eliminate chloride interference. Thus, COD vials are considered hazardous waste and must be handled and disposed of in an approved manner.
Do Not Dispose of COD Vial Contents Down the Drain!
Most pre-prepared COD vial vendors will have a return policy for used COD vials so that used vials can be returned to the vendor sealed in the original containers for proper disposal.
TOC (total organic carbon)
- The TOC test is gaining popularity because it only takes 5-10 minutes to complete.
- Like COD, the TOC test can be used to rapidly estimate BOD concentration once a consistent TOC to BOD ratio is established on a particular wastewater stream (See “Can I use my COD results to predict my BOD?” in the COD section).
- At the heart of the TOC test is a carbon analyzing instrument that measures the total organic carbon in a wastewater sample.
- Various heat and oxygen, ultraviolet radiation and chemical oxidant-based methods are available to measure TOC that are specific to the carbon analyzing instrument utilized.
- In the TOC test, organic carbon is converted to carbon dioxide (CO2) and typically measured with an infrared analyzer.
TOC Test Procedures
TOC test procedures are relatively simple and straight-forward, but are specific to the type of carbon-analyzing instrument utilized in the laboratory. Thus, no “typical” TOC procedure exists. The instrument manufacturer's procedures should be followed accurately to achieve the best results.
| APHA Standard Methods for TOC Measurement | |
| 5310 B. | High-Temperature Combustion Method |
| 5310 C. | Persulfate-Ultraviolet or Heated-Persulfate Oxidation Method |
| 5310 D. | Wet-Oxidation Method |
Concentration Versus Loading
Concentration (most often reported in wastewater samples as mg/L or ppm) tells how much of a substance (e.g., mg of BOD) is present in a known volume of wastewater (e.g., 1 Liter). However, concentration isn't the whole story since it does not tell how much (i.e., mass or weight) of a substance is going down the drain? commonly referred to as loading.
While wastewater pollutant concentrations are typically reported as mg/L or ppm, wastewater pollutant loadings are typically calculated and reported as pounds per day (lb/d) and are calculated using the following formula:
(Scroll right for more)
Flow
Million gallons per day (MGD)X Concentration
mg/L or ppmX 8.34
Weight (lb) of 1 gallon of water (MGD)=lb/day
The importance of understanding both concentration and loading can be highlighted by comparing two fictitious industrial plants. Plant A discharges effluent with a BOD level of 250 mg/L, while Plant B's discharge is 1000 mg/L to a city's sewer system. Simply looking at the difference in concentration between the two plants would lead us to believe that Plant B contributes a much higher amount of organics (four times as much) into the sewer. However, we need to take into account that Plant A is a large industrial manufacturer that discharges 1,000,000 gallons of wastewater per day (1.0 MGD), while Plants B is a much smaller facility only discharging 50,000 gallons each day (0.05 MGD). Plugging these values into the loadings formula gives the following results:
| (Flow) (Concentration) (8.34) = lb/day Plant A: (1.0 MGD) (250 mg/L) (8.34) = 2085 lb/day Plant B: (0.05 MGD) (1000 mg/L) (8.34) = 417 lb/day |
As this example shows, Plant B's BOD concentration is four times higher than Plant A. But, the loadings formula shows that Plant A produces five times more BOD by weight than Plant B.
O&G (oil and grease)
- O&G consists of a group of related constituents that are of special concern in wastewater treatment due to their unique physical properties and highly concentrated energy content.
- The term O&G (oil and grease) has become the popular term replacing the term FOG (fat, oil and grease), although both terms refer to the same wastewater constituents.
- O&G constituents in wastewater can come from plants and animals (e.g, lard, butter, vegetable oils and fats) as well as petroleum sources (e.g., kerosene, lubricating oils).
- O&G are generally hydrophobic (i.e., “water-hating”) and thus have low solubility in wastewater, resulting in relatively low biodegradability by microorganisms.
- O&G becomes more soluble (i.e., more easily dissolved) in wastewater at high temperatures and will form emulsions (i.e., oil-water mixtures) that will often separate back out of wastewater as temperatures become cooler; thus, O&G are notorious for causing sewer collection system problems (e.g., blockages, pump failures).
- WARNING! Since O&G adheres to plastic, only glass sample collection containers can be used to collect O&G samples!
O&G Test Procedures
- A clean flask is dried, cooled and weighed.
- A 1L wastewater sample is acidified (typically using hydrochloric or sulfuric acid) to a pH = 2.
- The acidified wastewater sample is then transferred to a 2L separatory funnel.
- 30 ml of the extraction chemical (e.g., n-Hexane) are then added to the funnel and the funnel is shaken vigorously for two minutes.
- The wastewater/extraction chemical layers are allowed to separate in the funnel (the lighter water layer will be on the top and heavier extraction chemical layer will be on the bottom). The bottom layer of extraction chemical is drained into the flask prepared in Step 1.
- Steps 4/5 are repeated twice more to extract O&G.
- The contents of the flask (i.e., the extraction chemical containing O&G) are then heated so that the extraction chemical is distilled into another container.
- The flask (containing the extracted O&G) is reweighed. The original weight of the flask is subtracted and the total O&G weight in mg is calculated. The results provide the O&G concentration in mg/L.
| APHA Standard Methods for O&G Measurement | |
| 5520 B. | Liquid-Liquid, Partition-Gravimetric Method1 |
| 5520 C. | Partition-Infrared Method |
| 5520 D. | Soxhlet Extraction Method |
| 1 EPA Approved Method | |
Selected References
APHA. 2005. Standard methods for the Examination of Water and Wastewater. 21st Edition. American Public Health Association, Washington, D.C.
CSUS. 1993. Operation of Wastewater Treatment Plants. Volume 2. 4th Edition. California State University, Sacramento, CA.
Metcalf & Eddy, Inc. 2003. Wastewater Engineering: Treatment and Reuse. 4th Edition. McGraw-Hill, New York, NY.
The University of Georgia Faculty of Engineering Outreach Service (EOS), working cooperatively with Bio & Ag Extension Engineering, provides engineering services and outreach to commercial, industrial and institutional clients throughout the state of Georgia. EOS services center on energy conservation, water conservation, water and air quality, solid waste and other environmental and sustainability issues. The overall goal of the EOS is to decrease the cost of doing business, increase environmental sustainability and competitiveness of the state's industries, lower costs for government agencies and facilities, and enhance economic development.
This publication was authored by Dr. Brian Kiepper of the University of Georgia Biological & Agricultural Engineering and Poultry Science Departments, and the Faculty of Engineering Outreach Service. Special thanks to the following individuals for input and review of this publication: Dr. Uttam Saha and Laura Daniel (Feed and Water Laboratory), Dr. Jason Evans (Carl Vinson Institute), Vaughn Berkheiser (Bio & Ag Engineering, Griffin Campus) at the University of Georgia; John Polanski, M.Ed., Minnesota Technical Assistance Program (MnTAP).
Status and Revision History
Published on Oct 14, 2010
Published with Full Review on Oct 01, 2013
Published with Full Review on Jul 07, 2016
Published with Full Review on Aug 18, 2022


























































