- How Soil pH Is Typically Measured
- Why Does Salt Affect pH Measurement?
- Is There a Better Way to Measure pH?
- Why Is a Stable pH Measurement Important?
- What Should I Expect in Measured Values of Soil pH with the New Method?

Soil pH is one of the most important measurements of soil fertility. It indicates whether a soil could contain toxic levels of aluminum and manganese, whether it may be low in bases such as calcium and magnesium, and therefore is lime is needed. The availability of other essential plant nutrients is also affected by pH. Therefore, knowing a soil's pH may help in diagnosing nutritional problems of agricultural crops and other plants.
How Soil pH Is Typically Measured
Most labs in the southeastern United States measure soil pH in a 1:1 mixture of soil:water using a pH electrode. Even when a soil’s acidity is unchanged, the measured soil pH may vary from year to year and within a growing season. This fluctuation occurs primarily because of changes in salt levels in the soil. Salt levels increase from adding nitrogen and potassium fertilizers, and from decomposition of soil organic matter and minerals. Salt levels decrease because of leaching rains. As a result of these interacting factors, there is considerable seasonal fluctuation in the salt content of many Georgia soils, since many of them are sandy and rainfall may be high.

Why Does Salt Affect pH Measurement?
A pH electrode is similar to a battery in that both of them require an electrolyte (acid/salt) to work. The amount of salt in the soil water mixture affects the pH value measured. Because the salt concentration varies in the soil by year and season, the pH values measured will also vary.
Is There a Better Way to Measure pH?
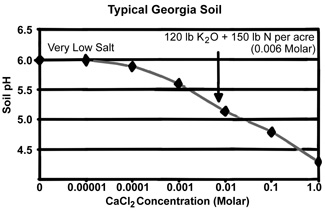
Yes. By measuring pH in a dilute salt solution, the pH readings will be more stable between years and during the season. We will use calcium chloride as our salt, because it is similar to the salts normally present in the soil. We will use a standard amount of salt (0.01 Molar) that is slightly greater than the amount found in Georgia soils when they are at their saltiest level after fertilizer application and organic matter decomposition in late spring. The way in which calcium chloride typically affects the measured pH in a typical Georgia soil is shown in the graph below. Soil pH drops nearly one pH unit from the very low salt condition (following heavy winter rains) to a salt content that would occur in late spring following fertilizer application (see example in the graph of 120 lb K2O plus 150 lb N per acre).
Why Is a Stable pH Measurement Important?
Lime is recommended for most Georgia crops only when soil pH is less than 6.0. In a particular year when winter rainfall is heavy, salts may be at a very low concentration, and soil samples collected at this time of year may, for example, read pH 6.1. In this case, lime would not be recommended. For the same soil in a dry winter, more salts may be present in the soil and the pH might read 5.8 and lime would be recommended, even though lime may be needed in both cases. By measuring pH in a salt solution, the salt content when measuring pH would be very close to the same from year to year, resulting in more stable soil pH readings between years and during the year. A more stable seasonal pH measurement will result in lime being recommended when needed, and thus avoiding situations where lime is not recommended but should be.
What Should I Expect in Measured Values of Soil pH with the New Method?
With the new method, soil pH readings will be lower. We have found that pH measured in calcium chloride is an average of about 0.6 units lower than when measured in water but, as noted above, it will be more consistent from year to year and within a year. The laboratory will report only one pH value to the client, which will be the equivalent water pH (salt pH + 0.6). The table below illustrates how equivalent water pH is calculated.
| pH in calcium chloride 5.3 |
Add 0.6 |
= Equivalent water pH 5.9 |

The value of pH we report will therefore relate well to published values of recommended pH for various crops. The pH will also be more stable for better comparisons across years and within a year (seasonal).
For those soils with a salt pH less than 5.4, the Lime Buffer Capacity (LBC) will be used to determine the soil's lime requirements. The new LBC procedure is described in another soil testing circular, Measurement of Lime Buffer Capacity.


Status and Revision History
Published on Oct 29, 2004
Published with Minor Revisions on Aug 30, 2006
Published on Sep 14, 2009
Published with Full Review on Sep 01, 2012


























































