- Soil sample collection
- Action threshold levels
- Sample collection timing
- General management options
- Corn
- Cotton
- Peanut
- Soybean
- Apples, Pecans, Blueberries, Brambles
- Canola and Rapeseed
- Tobacco
- Vegetables (Commercial)
- Vegetables (Home Garden)
- Ornamentals (Landscape)
- Peach (Pre-plant Thresholds, Orchard Establishment Only)
- Turf (Commercial, Golf Courses)
- Turf (Home Lawns)
- Pesticide Precautions
The University of Georgia Extension Nematology Laboratory is part of the Department of Plant Pathology in the College of Agricultural and Environmental Sciences. The laboratory's primary responsibility is to provide nematode assay results to Georgia's county Extension offices so Extension agents and specialists can provide the necessary educational materials to Georgians involved in agricultural efforts. This service provides growers with the information they need to optimize nematode management for their individual situations. Optimizing pest management is a significant step toward sustainable and environmentally sound agricultural production.
Sending soil samples to the Extension Nematology Laboratory for analysis is only the first step in effective nematode management. Once the lab determines which nematode species are present and at what densities, you must decide if control measures are necessary and, if so, which measures should be employed. Every situation is unique and there are no hard rules to follow, but some very general guidelines can be given.
This publication is designed to help county agents, Extension specialists and growers formulate and implement nematode management recommendations after sending a soil sample and receiving a nematode assay report. Guidelines presented here are not absolute and personal judgment and experience should always be part of the decision-making process. Additional information about diagnosing and controlling specific nematode problems may be found in crop-specific publications from UGA Cooperative Extension.
Soil sample collection
It is very important that the soil sample be truly representative of the area sampled. The only way to ensure this is to collect the sample from many spots around the field rather than from only one or two spots. For example, if a problem area is being sampled, collect soil from the margin of the affected area to ensure the presence of a high nematode population (Fig. 1).
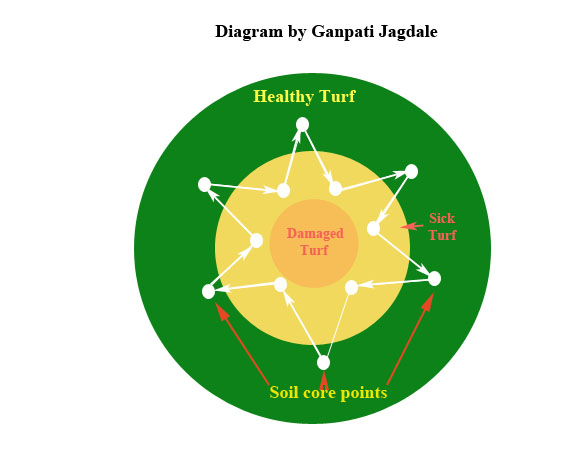 Figure 1. Diagram by Ganpati Jagdale
Figure 1. Diagram by Ganpati JagdaleLarge areas (fields, golf course fairways and rough areas), small areas (home lawns, recreational parks and golf course greens) and ornamental plants/ fruit trees should all be sampled in a systematic zigzag pattern (Figs. 2, 3, 4 and 5).
 Figure 2. Soil sampling pattern for field crops or golf course
Figure 2. Soil sampling pattern for field crops or golf course Figure 3. Soil sampling pattern for small area or golf course greens
Figure 3. Soil sampling pattern for small area or golf course greens Figure 4. Soil sampling pattern for row crops
Figure 4. Soil sampling pattern for row crops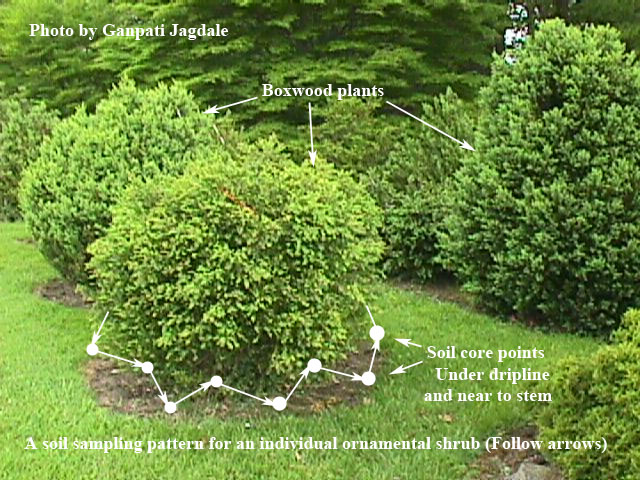 Figure 5. Soil sampling pattern for an individual ornamental shrub/bush or fruit trees
Figure 5. Soil sampling pattern for an individual ornamental shrub/bush or fruit treesWhen the sample is taken, soil moisture should be about right for good seed germination. Do not take samples from extremely dry soil. DO NOT ALLOW SAMPLES TO GET HOT OR DRY. Allowing samples to sit in direct sunlight or in a hot vehicle for even a short time can kill the nematodes in the sample. Nematodes must be alive for the extraction procedure to work. Killing the nematodes in the sample may cause failure to detect nematodes when they are actually present. Your county Extension office will send the samples to the Extension Nematology Laboratory for assay.
Action threshold levels
An action threshold is the nematode population density at which control measures must be implemented to prevent economic loss due to nematodes. Threshold levels in Georgia are based on available research and field observations and may differ from thresholds used in nearby states. Thresholds may vary due to differences in sample collections methods, nematode assay methods, soil types, climate, etc. Some differences may result from simple differences of opinion. Where possible, our threshold levels are based on thorough research, but when necessary hard data are lacking, we choose to establish a threshold value anyway because we believe an educated estimate is better than no guidance at all.
Nematodes that are believed to cause economic losses are listed for each crop with an action threshold (Fig. 6). For each crop, there may be certain plant-parasite nematode species present in a sample that are not known to cause damage even at high levels. If a species has not been shown to cause a loss of yield or quality, no threshold is given and it is assumed that no management is necessary even if that species is present in high levels. Once a damaging species is identified in a field, it will be present in all subsequent years, although population levels will fluctuate. Proper sampling of such fields is critical to ensure that the management system being used is keeping known nematode pests in that field below threshold levels.
 Figure 6. Different types of plant-parasitic nematodes that cause economic loss to different types of crops in Georgia crops
Figure 6. Different types of plant-parasitic nematodes that cause economic loss to different types of crops in Georgia cropsAccurate and detailed records should be kept for each field to track where each nematode species occurs and where populations tend to be highest. In fields where a damaging nematode species is detected at sub-threshold levels, it is possible that "hot spots" with above-threshold levels can occur. If such hot spots are identified, localized nematicide applications may be profitable where application to the whole field would not be.
It is common for a sample to contain two or more nematode species each at sub-threshold levels. In these situations, some common sense must be exercised. As an example, if two species are each present at 90 percent of their threshold levels, then management tactics probably should be implemented. This does not imply that nematode damage is necessarily additive. You cannot simply add up the threshold percentages for all species present to see if it surpasses 100 percent. That is, if four species are each present at 30 percent of their respective threshold levels, do not assume that their combined effect warrants control measures. If nematodes are below but approaching threshold levels, check and correct other limiting factors such as pH, fertility, hardpan, etc., before implementing nematode control measures. Healthy plants that are not under other significant stresses are better able to withstand nematode feeding pressure. Conversely, plants that are under significant stress (such as low pH soil with a hardpan and inadequate moisture) may be damaged by nematode population levels below those needed to damage otherwise healthy plants.
Nematode control is usually based on economic value where one considers both the potential gain from controlling nematodes and the potential loss from not controlling them. As the value of the crop increases, controlling nematodes is more likely to provide economic benefit and thresholds are likely to be lower; low-value crops may not be able to recoup the cost of control measures. The cost and the potential benefits of control will determine which control measures (rotations, nematicides, etc.) are preferable.
If either the cost of control or the value of the crop changes significantly, control recommendations and threshold levels may need to be re-evaluated. Threshold levels given in this guide are based on samples taken from shortly before until shortly after harvest for row crops. For perennial crops such as turf and peaches, collect samples when both plants and nematodes are active. In established peach orchards, collect samples in the fall (September-October) to determine root-knot nematode population levels and in the spring (February-April) to determine ring nematode population levels.
Thresholds and recommendations for peaches in this guide are for orchard establishment only and should not be used to make recommendations for established orchards. Optimum sample times for the crops in this guide are given in Table 1.
| Table 1. Optimum time to take samples for nematode assay from various Georgia crops. | |
| Crop | When to Sample |
| Cotton | October-November |
| Fruit Orchards (except peaches) | September-October |
| Peaches | September-October (root-knot) February-April (ring) |
| Peanuts | September-October |
| Soybeans | September (group IV) October (groups V, VI) November (group VII) |
| Tobacco | July |
| Turfgrass | |
| — warm season | June-August |
| — cool season | September-October, April |
| Vegetables | August-September |
Sample collection timing
The timing of collecting nematode samples is important because nematode populations fluctuate throughout the year. Nematodes may be undetectable during the winter and early spring but increase to very high levels before harvest; following harvest, population levels may decline precipitously. Sampling when population densities are high decreases the risk of failing to detect a damaging species.
Nematode populations overwinter primarily as eggs. "Winter" can loosely be defined as December through March when most Georgia soils are too cold to support active root growth of warm-season crops. Unfortunately, typical laboratory assays do not detect nematode eggs, so samples collected in the winter frequently fail to detect nematodes when there are actually many nematode eggs present. Samples collected during the winter are of little value for comparison to threshold levels. The only value that such samples can have is to identify certain species as being present in the field. It must be noted that failure to detect a species does not necessarily mean that it is not present; the species may be present at low levels that the random sample missed or it may be present only as eggs, which the assay cannot detect. Because of these limitations, do not collect samples during the winter.
General management options
Nematode management options are presented for each crop. The recommendations given here are brief and are meant to be taken as a general guide and not as definitive, current control recommendations. For the most current recommendations, contact your county Extension office. More details about management options can be found in crop-specific Extension publications as noted in the comments sections under each crop heading. Generally, resistant varieties will be preferable to chemical control if such varieties are available, but they must be used as part of a rotation and not grown every year or they may lose their efficacy as a management tool. New varieties with varying levels of resistance are released each year. For some crop/nematode combinations, resistance is not yet available; if resistant varieties become available, they should be used as nematode management tools.
Crop rotations with non-host plants may provide benefits in addition to nematode control and should be considered if economically acceptable. Consider multi-year rather than year-to-year profits when determining if crop rotations are economically acceptable. Significant yield increases (and increased profits) may occur following rotation with a non-host; even if the rotation crop is not very profitable, increased profits from the primary crop may economically justify the rotation sequence.
Combining the use of a nematicide with resistant varieties or non-host crop rotations may decrease nematode population densities, which may increase the effectiveness of chemical control and allow the use of lower nematicide rates. For current resistant variety and cropping sequence recommendations, contact your county Extension office.
Root-knot nematode identification
Current nematicide recommendations can be found in the Georgia Pest Management Handbook. For some crops, the comments section notes that identifying root-knot species is very important. The Extension Nematology Laboratory can speciate root-knot nematodes if requested to do so, but the procedure is not routine. Root-knot speciation requires mature root-knot females, which are found in the galls on the roots. If speciation is requested, root samples must be sent to the lab with the soil sample.
Take samples from an area known to be infested with nematodes. The root sample should contain as many root-knot galls as possible (Fig. 7), since not all of the galls will contain useful females. Do not send roots that have deteriorating galls; the nematodes in those galls will probably be too old to use for speciation. Once a root-knot species is identified in a field, it will always be present and additional speciations will not be necessary unless a different crop is to be grown.
 Figure 7. Fresh galls for extraction of root-knot nematode females
Figure 7. Fresh galls for extraction of root-knot nematode females
Corn
| Nematode | Assay Result | Recommendation |
| Stubby-Root (Paratrichodorus spp.) |
1-9 | No control necessary. |
| 10-39 | This number of nematodes may begin to cause damage. Correct any pH, fertility or hardpan conditions. Nematicides may be beneficial. | |
| 40 or more | Corn crop will be affected. Use a nematicide or rotate to a non-host. | |
| Sting (Belonolaimus longicaudatus) |
1 or more | Corn crop will be affected. Use a nematicide or rotate to a non-host. |
| Lance (Hoplolaimus galeatus) |
60 or more | Corn crop will be affected. Use a nematicide or rotate to a non-host. |
| Lesion (Pratylenchus zeae) |
200 or more | This number of nematodes may begin to cause damage. Correct any pH, fertility or hardpan conditions. Nematicides may be beneficial. |
| Root-knot (Meloidogyne spp.) |
1-49 | No control necessary. |
| 50-99 | This number of nematodes may begin to cause damage. Correct any pH, fertility or hardpan conditions. Nematicides may be beneficial. | |
| 100 or more | Corn crop will be affected. Use a nematicide or rotate to a non-host. | |
| Stunt (Tylenchorhynchus spp.) |
200 or more | This number of nematodes may begin to cause damage. Correct any pH, fertility or hardpan conditions. Nematicides may be beneficial. |
Comments:
Corn is a relatively low-profit crop in Georgia, and the costs of nematicides may preclude their use in nematode management unless the crop is grown for on-farm use. Some soil-applied insecticides have nematicidal properties, so insect and nematode control may be achieved with a single chemical application. The amount of nematode control obtained with soil-applied insecticides will be influenced by the application method and rate used. If the value of a nematicide application is in question, it may be applied to small sections of the field on a trial basis. Consult the Georgia Pest Management Handbook for current pesticide recommendations.
Subsoiling under the row may reduce plant stress and improve the plant's ability to withstand nematode feeding pressure. Generally, anything that reduces plant stress will help minimize crop loss due to nematodes. Minimizing nutrient, disease, insect and moisture stress will also help minimize nematode damage.
Cotton
| Nematode | Assay Result | Recommendation |
| Southern root-knot (Meloidogyne incognita) | 1-99 |
Cotton crop will be affected. Use a tolerant variety. |
| 100 or more | Use a tolerant variety and a nematicide. Consider implementing a crop rotation sequence with a non-host. | |
| Lance (Hoplolaimus columbus) |
1-79 | This number of nematodes may begin to cause damage. Correct any pH, fertility or hardpan conditions. Nematicides may be beneficial. |
| 80 or more | Cotton crop will be affected. Use a nematicide. Implement a crop rotation sequence with a non-host. | |
| Reniform (Rotylenchulus reniformis) |
1-249 | This number of nematodes may begin to cause damage. Correct any pH, fertility or hardpan conditions. Nematicides may be beneficial. |
| 250 or more | Cotton crop will be affected. Use a nematicide. Implement a crop rotation sequence with a non-host. | |
| Sting (Belonolaimus longicaudatus) |
1 or more | Cotton crop will be affected. Use a nematicide. Implement a crop rotation sequence with a non-host. |
Comments:
The peanut root-knot and javanese root-knot nematodes do not need to be controlled in cotton fields because these nematodes cannot feed on cotton roots and cannot damage the crop. There are two commonly found species of lance nematode in Georgia, but only the Columbia lance nematode is a problem on cotton. Cultivars tolerant to the southern root-knot nematode are available and may reduce the need for nematicides or allow the use of lower rates. Root-knot nematodes will still reproduce on these varieties, but yield may not be reduced sufficiently to justify the use of nematicides. Nematicides must be used to control above-threshold levels of lance, reniform and root-knot nematodes. If the value of a nematicide application is in question, it may be applied to small sections of the field on a trial basis. Consult the Georgia Pest Management Handbook for current pesticide recommendations. If left uncontrolled for a single growing season, root-knot and reniform nematodes can increase to very high levels that can cause significant problems in subsequent crops. A cotton-peanut crop rotation can be used to manage these nematodes. Other rotations may also be useful if economically justified.
The stubby-root nematode (Paratrichodorus spp.) can parasitize cotton, but it is not known how many of these nematodes must be present to cause yield reductions. If this nematode is present at high levels and damage is evident, or you suspect that yields have been reduced, nematicide use may be beneficial.
Sub-soiling under the row may reduce plant stress and improve a plant's ability to withstand nematode feeding pressure. Generally, anything that reduces plant stress will help to minimize crop loss due to nematodes. Minimizing nutrient, disease, insect and moisture stress will also help minimize nematode damage.
Peanut
| Nematode | Assay Result | Recommendation |
| Peanut root-knot (Meloidogyne arenaria) |
1-9 | This number of nematodes may begin to cause damage. Correct any pH, fertility or hardpan conditions. Nematicides may be beneficial. |
| 10 or more | Peanut crop will be affected. Use a nematicide. Implement a crop rotation sequence with a non-host. | |
| Southern root-knot (M. incognita) |
1 or more | No control necessary. |
| Lesion (Pratylenchus brachyurus) |
300 or more | This number of nematodes may begin to cause damage. Correct any pH, fertility or hardpan conditions. Nematicides may be beneficial. |
Comments:
The peanut root-knot nematode is the most widespread and damaging nematode pest of peanut in Georgia, but the southern root-knot nematode does not parasitize peanut. For this reason, it is important to identify which species of root-knot is present in the field. If the species cannot be identified, assume that any root-knot present is the peanut root-knot nematode and act accordingly. If the unidentified species is actually the southern rootknot nematode, then the cost of nematicide application will be an unnecessary expense, but the potential loss from an unidentified root-knot species is too great a risk to take. If samples are collected in the fall (September- October) prior to planting peanuts, the host plant in the field at the time of sampling may indicate which species is present. It may be assumed that root-knot nematodes from cotton fields are the southern root-knot nematode. If collected from a peanut field, it may be assumed that the species is the peanut root-knot nematode.
Subsoiling under the row may reduce plant stress and improve a plant's ability to withstand nematode feeding pressure. Generally, anything that reduces plant stress will help minimize crop loss due to nematodes. Minimizing nutrient, disease, insect and moisture stress will also help minimize nematode damage. If the value of a nematicide application is in question, it may be applied to small sections of the field on a trial basis. Consult the Georgia Pest Management Handbook for current pesticide recommendations.
Currently, there are no cultivars with resistance to the peanut root-knot nematode. If resistant varieties are developed, they should be incorporated into a nematode management program. No varieties resistant to lesion nematodes are available.
Soybean
| Nematode | Assay Result | Recommendation |
| Soybean Cyst (Heterodera glycines) |
1 or more | Soybean crop will be affected. Implement crop rotation with non-hosts, susceptible and resistant cultivars. |
| Southern root-knot (Meloidogyne incognita) |
1-59 | This number of nematodes may begin to cause damage. Correct any pH, fertility or hardpan conditions. Use a resistant variety. |
| 60 or more | Soybean crop will be affected. Use a resistant variety. | |
| Peanut root-knot (M. arenaria) |
1-9 | This number of nematodes may begin to cause damage. Correct any pH, fertility or hardpan conditions. Use a resistant variety. |
| 10 or more | Soybean crop will be affected. Use a resistant variety. | |
| Javanese root-knot (M. javanica) |
1-9 | This number of nematodes may begin to cause damage. Correct any pH, fertility or hardpan conditions. Use a resistant variety. |
| 10 or more | Soybean crop will be affected. Rotate with a non-host. | |
| Lance (Hoplolaimus columbus) |
1 or more | Soybean crop will be affected. Rotate with a non-host. |
| Sting (Belonolaimus longicaudatus) |
1 or more | Soybean crop will be affected. Rotate with a non-host. |
| Reniform (Rotylenchulus reniformis) |
1 or more | Soybean crop will be affected. Rotate with a non-host. |
Comments:
Soybean cyst-resistant varieties should never be planted year after year or new races of the nematode will develop that are capable of reproducing on the resistant varieties. Contact your county Extension office to identify cultivars that are resistant to various races of soybean cyst nematode. Resistant varieties are also available for the southern root-knot, peanut root-knot and reniform nematodes.
To manage root-knot nematode populations, identification of the species in the field is critical. Cultivars have varying levels of resistance to different root-knot species, and a cultivar resistant to one species may not be resistant to another species. By identifying which species are present in the field, cultivar selection can be targeted to control those species that are present. If the species are unidentified, a cultivar that is resistant to all three species must be used, and this significantly restricts cultivar selection. If cotton was the crop present at the time of sampling, the species is probably the southern root-knot nematode (M. incognita). If peanut was the crop present at the time of sampling, the species is probably the peanut root-knot nematode (M. arenaria). If the species cannot be determined by the crop present at the time of sampling, the Extension Nematology Laboratory can identify the species through a time-consuming and expensive laboratory procedure. Because of the time and costs involved, species identification with this procedure is not routine but will be carried out if requested.
Soybeans are a relatively low-profit crop in Georgia and the costs of nematicides may preclude their use in nematode management. If the value of a nematicide application is in question, it may be applied to small sections of the field on a trial basis. Consult the Georgia Pest Management Handbook for current nematicide recommendations.
Apples, Pecans, Blueberries and Brambles
There are no known nematode problems on these crops in Georgia. Little work has been done to determine which nematode species parasitize these crops. Yield loss caused by nematodes has not been reported in Georgia.
Canola and Rapeseed
Some varieties of canola and rapeseed have been reported to be hosts for the southern root-knot nematode (Meloidogyne incognita), whereas other varieties have been reported to be very poor hosts. Because canola and rapeseed are grown as winter crops, when nematodes are less active in Georgia, the southern root-knot nematode has not been shown to cause yield loss. Under typical growing conditions in Georgia, little galling or nematode reproduction has been observed on canola and rapeseed. Nematode populations could, however, increase on canola and rapeseed under the right environmental conditions. Such an increase would be uncommon and would be unlikely to damage the canola or rapeseed crop but could cause problems if a susceptible crop was planted the following spring. Though this has not yet been observed in Georgia, it is possible. To protect subsequent crops, canola and rapeseed roots should be checked for galling and a soil sample should be taken in the spring to determine if reproduction has occurred.
Tobacco
| Nematode | Assay Result | Recommendation |
| Lesion (Pratylenchus brachyurus) |
1-99 | This number of nematodes may begin to cause damage. Correct any pH, fertilizer or hardpan conditions. Rotate with a non-host. |
| 100 or more | Tobacco crop will be affected. Use a nematicide. |
Comments:
Root-knot nematodes (RKN) are the major tobacco nematodes in Georgia. Soil assays taken from crops other than tobacco the season before planting tobacco have been found NOT to provide reliable information for scheduling nematicide treatment. Assays of soil collected from tobacco may indicate an active RKN population, but they cannot tell you which RKN species is present or how much damage has occurred on this crop or may occur on the next tobacco crop. Examination of root systems between topping and harvest is the best way to evaluate tobacco for RKN damage. To fully evaluate a field for RKN damage potential, it is necessary to compare the root systems of nematicide-treated and untreated tobacco. Untreated tobacco can be a few randomly placed rows that represent the field. Roots of these plants will be compared to the roots of plants in adjacent treated rows. Every 20th to 50th plant (examine 15-20 plants per row) in a row should be dug (not pulled up) and examined for root galls. Estimate the percent of each plant's root system where galls occur and compare the results for treated and untreated rows (see table below). Sampling only treated tobacco is better than nothing, but it will not be as informative as comparing treated to untreated tobacco. Use a nematicide if active RKN populations are present.
Crop rotation alone cannot be counted on to provide good control every year. Crop rotation can, however, help improve nematicide performance by lowering the initial RKN population. For crop rotations to be effective, you must use crops that are non-hosts or very poor hosts for the RKN species present. Galled roots can be used by the Extension Nematology Laboratory to speciate RKN.
| Root Examination Guidelines for Tobacco | ||
| % Root Galling1 | ||
| Treated | Untreated | Comments2 |
| <5% | >35% | Damage potential for this field is moderate to high. |
| <5% | <5% | Damage potential for this field is low. Nematicide may not be needed depending on how rotational crops are used. |
| 20-30% | 25-50% | Damage potential for this field is moderate to high. Nematicide treatments are not providing expected control. Check all aspects of treatment program. |
| 20-30% | >75% | Damage potential in this field is very high. Nematicide program may be doing as well as can be expected under these conditions. |
| <5% | — | Without untreated tobacco for comparison, you cannot be certain if the damage potential is very low or the nematicide has done a very good job. |
| 20-30% | — | You cannot be sure if the damage potential for this field is high or if the nematicide is not providing expected control. |
| >30% | — | Moderate potential for damage. Nematicide program is not providing expected control |
| 1% galling means the average % of individual root systems containing galls and not the % of plants with some galls in the root systems. 2Some comments suggest nematicide failure. This almost never occurs. In nearly all cases, poor nematicide performance is caused by poor nematicide application. |
||
Vegetables (commercial)
| Nematode | Assay Result | Recommendation |
| Root-knot (any species) (Meloidogyne spp.) | 1 or more | Vegetable crop will be affected. Use a resistant variety or a nematicide. Rotate with a non-host. |
| Sting (Belonolaimus longicaudatus) |
1 or more | Vegetable crop will be affected. Use a nematicide. Rotate with a non-host. |
| Reniform (Rotylenchulus reniformis) |
1 or more | Vegetable crop will be affected. Use a nematicide. Rotate with a non-host. |
| Cyst (Heterodera spp.) |
1 or more | Vegetable crop will be affected. Rotate with a non-host. |
Comments:
The use of nematicides in vegetables is usually justified economically. Generally, non-fumigant nematicides will provide effective control for low levels of southern root-knot nematodes. Fumigant nematicides work better for peanut root-knot, javanese root knot and high levels of southern root-knot nematodes. Consult the Georgia Pest Management Handbook for current nematicide recommendations. Rotations with non-host crops can aid significantly in nematode management and should be used where possible.
Varieties resistant to root-knot nematodes are available for some vegetables and should be used where these nematodes are a problem. If resistance is available for a specific vegetable, it is usually effective against southern root-knot, peanut root-knot and javanese root-knot. Contact your county Extension office for information about which vegetable species and varieties have resistance to root-knot nematodes.
Not all vegetable crops are susceptible to all species of root-knot nematode -- but most are. Sweet corn is susceptible to the southern and javanese root-knot nematodes, and sweet potato is susceptible to the southern and northern root-knot nematodes. If these crops are to be grown, it is important to know which species of root-knot is present in the field. By identifying which species are present, rotations with resistant or non-host crops can be used as nematode management tools. If the species are unidentified, nematicides must be used and resistant varieties cannot be used effectively. Root-knot species sometimes may be identified by the crop that was growing when the sample was collected. For example, if cotton was the crop present at the time of sampling, the species is probably the southern root-knot nematode. If the species cannot be determined by the crop present at the time of sampling, the Extension Nematology Laboratory can identify the species through a time-consuming and expensive laboratory procedure. Because of the time and costs involved, species identification with this procedure is not routine but will be carried out if requested.
Cyst nematodes usually have a fairly narrow host range and can be controlled easily by rotating with a non-host. Root-knot, sting and reniform nematodes can affect most vegetable crops, so these nematodes must usually be controlled by nematicides.
Generally, anything that reduces plant stress (such as minimizing nutrient, disease, insect and moisture stress) will help minimize crop loss due to nematodes. The exceptions are crops such as carrot, where nematode damage results in an unmarketable product rather than simply a yield reduction.
Vegetables (home garden)
| Nematode | Assay Result | Recommendation |
| Root-knot (any species) (Meloidogyne spp.) |
1 or more | Vegetable crop may be affected. Use a resistant variety if available. Rotate with a non-host. |
| Sting (Belonolaimus longicaudatus) |
1 or more | Vegetable crop may be affected. Rotate with a non-host. |
| Reniform (Rotylenchulus reniformis) |
1 or more | Vegetable crop may be affected. Rotate with a non-host. |
| Cyst (Herterodera spp.) |
1 or more | Vegetable crop may be affected. Rotate with a non-host. |
Comments:
The same nematode species that cause problems on commercial vegetables can also be pests in home gardens; however, there are no nematicides that can be used by homeowners. Currently, licensed pesticide applicators can still use metam sodium, a pre-plant fumigant, on home gardens, but this chemical requires important safety precautions and may carry significant legal liabilities. Therefore, chemical control is not a viable option in home vegetable gardens.
If you have root-knot nematodes, choose resistant varieties if they are available. For any nematode species, rotate with a non-host, if possible. A non-host is a plant on which the nematode cannot feed. If there is space to move the garden spot around, leaving an area fallow for an entire growing season can reduce nematode populations. Roto-till fallow areas once a month to prevent any weed growth; weeds can support nematode reproduction, which would negate the benefit of fallowing the field.
If the garden is in a sunny spot, heating the soil with solar energy (called soil solarization) may be beneficial. This procedure involves roto-tilling the garden and then tarping the area with a plastic film that traps natural solar radiation in the soil. This can raise the temperature of the soil enough to kill most of the nematodes present. Solarization requires weeks of intense solar radiation to be effective. In Georgia, the most effective time to use solarization is in June and July, when temperatures and solar radiation are high. Since the garden cannot be planted during this time, soil solarization may be a good management tactic to combine with fallowing.
The less stress plants are under, the better able they are to withstand nematode parasitism. Anything that can be done to reduce the overall stress on the plants will help alleviate pressure from nematodes. Watering the plants deeply and less frequently may encourage the growth of deeper roots, which can help minimize nematode problems. Proper management of diseases and insects can reduce stress and help reduce damage from nematodes. Nutrient deficiencies and soil compaction can inhibit root development and increase plant sensitivity to nematode damage. Nematode damage in home vegetable gardens is more common in sandy soils than in heavier soils.
Soil amendments may improve plant health and vigor, but they must be incorporated as deeply as possible before seeding or transplanting. Any amendment that improves soil composition, moisture-holding characteristics or physical characteristics will help produce healthier plants. Compost and pine bark are two common amendments that generally improve plant health and soil conditions. Pine bark, which works best if very fresh and ground into small pieces, has been reported to help suppress root-knot nematode damage.
Currently, no biological control agents provide consistent nematode control in home gardens. The use of suppressive plants such as French marigolds as a rotation crop may be beneficial, though by itself this is not likely to provide sufficient nematode control. Rotation crops must be grown in the absence of any host plants (including weeds) to be effective. If allowed to produce seeds, this year's rotation crop may become next year's weed problem. If the effectiveness of a control measure is in question, try it on a small section of the garden on a trial basis, but remember to leave an adjacent section of garden untreated so you will have something to compare the treatment to.
Ornamentals (landscape)
No thresholds have been established for ornamental plants. In Georgia, most of the identifiable nematode problems on landscape plants involve a species of either root-knot nematode or lesion nematode. Other nematode species such as Ditylenchus (the stem and bulb nematode) and Aphelenchoides (the foliar nematode) may also cause significant damage on ornamentals, but outbreaks of these nematodes are not common in Georgia. When outbreaks do occur, they are usually found in commercial nurseries where proper sanitation can eliminate the problem.
In high-visibility, high-value, critical plantings, it may be best to take a qualitative approach and assume a threshold of one nematode; if nematodes are present, they should be managed to prevent damage from occurring. In less critical plantings, the cost of management must be weighed against the potential damage. Threshold levels should be very low for perennial plants, because nematodes will have plenty of time to build up to damaging levels. For short-lived annuals, the concern is more likely to be preventing the nematode population from increasing to levels that can damage subsequent plantings. Remember that bulb crops are perennials.
No post-plant nematicides are labeled for use on outdoor landscapes. A nematode assay should be conducted prior to planting so pre-plant management options can be used if necessary. Depending on the location, options may include non-host or resistant plants, soil amendments, physical barriers (such as lined, raised beds) or preplant soil fumigation.
The less stress the plants are under, the better able they are to withstand nematode parasitism. Anything that can be done to reduce the overall stress on the plants will help alleviate pressure from nematodes. Watering the plants deeply and less frequently may encourage the growth of deeper roots, which helps minimize nematode problems. Proper management of diseases and insects can reduce stress and help reduce damage from nematodes. Nutrient deficiencies and soil compaction can inhibit root development and increase plant sensitivity to nematode damage.
Amendments that improve the soil composition, moisture-holding characteristics or physical characteristics will help produce healthier plants, but they must be incorporated as deeply as possible before planting. Compost and pine bark are two common amendments that generally improve plant health and soil conditions. Very fresh pine bark, which works best if ground into small pieces, has been reported to help suppress root-knot nematode damage.
Peach (pre-plant thresholds — orchard establishment only)
| Nematode | Assay Results | Recommendations |
| Ring (Criconemella xenoplax) |
1 or more OR any history of peach tree short life | Select another site OR prepare and fumigate this site by November 1. DO NOT use Nemaguard rootstock on this site. DO NOT prune trees on this site between November 1 and February 1. |
| Root-knot (M. arenaria, M. hapla, M. javanica) |
1 or more OR any history of root-knot on other crops | Select another site OR prepare and fumigate this site by November 1. |
| Root-knot (M. incognita) |
1 or more OR any history of root-knot on other crops | Select another site OR prepare and fumigate this site by November 1 OR south of U.S. Highway 2801, use Nemaguard rootstock2; north of Highway 280, use Guardian rootstock. |
| Lesion (Pratylenchus vulnus) |
1 or more | Select another site OR prepare and fumigate this site by November 1. |
| 1 U.S. Highway 280 is given as a rough dividing line to separate the northern two-thirds of the state from the southern third. U.S. Highway 280 runs approximately from Columbus to Savannah. 2 DO NOT use Nemaguard rootstock on ANY site where C. xenoplax is present OR where there is ANY history of peach tree short life. |
||
Comments (orchard establishment only):
Root-knot nematodes can be a limiting factor in establishing an orchard. Once the trees become well established (5-7 years), RKN do not seem to cause significant damage. RKN levels can be reduced by preplant rotations. In the fall (September-October), 18 months prior to planting, collect soil samples to determine if RKN are present. Do not sample extremely dry soil. If RKN are present, the species must be determined. The Extension Nematology Laboratory can speciate RKN for you. If RKN are present, use a rye winter cover crop followed by a summer fallow and another rye winter cover crop prior to transplanting to reduce nematode populations prior to fumigation. If is very important that the fallow field be kept weed-free or nematodes may be able to reproduce on the weeds present and limit the effectiveness of fallowing. Other rotations may also be effective.
If the RKN species present is Meloidogyne incognita, resistant rootstocks are available and should be used. Nemaguard rootstock is resistant to M. incognita, but it does not have good cold tolerance and is susceptible to peach tree short life, so do not use it in the northern two-thirds of the state. Guardian rootstock also is resistant to M. incognita, has good cold tolerance, and appears to have tolerance to ring nematodes and peach tree short life. Contact your county Extension office for rootstock recommendations for your area. Ring nematodes play a significant role in the peach tree short life complex and must be controlled. A preplant non-host or fallow rotation may be used to reduce ring nematodes and should be used if peach tree short life is a concern. Pre-plant fumigation can significantly reduce ring nematode populations and should be used if peach tree short life if a concern. If the value of a nematicide application is in question, it may be applied to small sections of the field on a trial basis.
Completely subsoiling the field (in two perpendicular directions) may improve root growth, reduce plant stress and improve the peach trees' ability to withstand nematode feeding pressure.
Turf (commercial — golf courses)
| Nematode | Assay Result | Recommendation |
| Sting (Belonolaimus longicaudatus) | 10 or more | Turf will be affected. Use a nematicide. |
| Lance (Hoplolaimus galeatus) | 60 or more | Turf may be affected. Use a nematicide. |
| Root-knot (Meloidogyne spp.) | 80 or more (primarily bermudagrass) | Turf may be affected. Use a nematicide. |
| Stubby-root (Paratrichodorus spp.) | 100 or more | Turf may be affected. Use a nematicide. |
| Ring (Criconemella ornata) | 500 or more | Turf may be affected. Use a nematicide. |
| Stunt (Tylenchorhynchus spp.) | 1,000 or more | Turf may be affected. Monitor closely and apply a nematicide if turf shows symptoms of nematode damage. |
Comments:
Nematicides remain a key tool in nematode management in putting greens but, to be most effective, they should be used in combination with proper turf management. Nematodes can cause significant damage to turf roots under the best of conditions, but turf that is suffering other stresses is more likely to show symptoms of damage. Proper pH, fertility, insect and water management can allow the turf to better withstand nematode damage. Correct any stress from these factors before nematicides are used. Mowing height is an important stress on golf turf; fairways should be able to withstand a specific number of nematodes better than putting greens.
Avoid nematicide use on bentgrass greens when turf is suffering from extreme environmental stress in the summer. The best time to apply nematicides to cool season grasses is in the fall or spring (not in December, January or February). Warm season turf may be treated with nematicides at any time, unless it is to be overseeded with a cool season grass. Do not apply nematicides within six weeks of overseeding. Repeated use of nematicides on turf has been reported to lead to enhanced microbial degradation and a loss of nematicide effectiveness. This happens when microorganisms in the soil develop the ability to degrade the chemical before it has been in the soil long enough to kill the nematodes. This can lead to an inability to manage nematode populations on the golf course. To prevent this from happening, never apply a nematicide more frequently or at higher rates than the label allows, and use a nematicide only when it is truly needed. Too much organic matter (such as a heavy thatch layer) can also reduce the effectiveness of nematicides by binding to the chemical, though this should not render a nematicide ineffective. On very high-sand content greens, excessive rain or irrigation may reduce nematicide effectiveness by washing a large proportion of it through the green before it can kill the nematodes. Normal irrigation or a single thunderstorm should not affect nematicide efficacy. Turf should be re-sampled six to eight weeks after a nematicide application to ensure that nematode populations have been reduced. The best time to sample turf is during periods of optimum growth: fall (September, October) and spring (April) for cool season turf and summer for warm season turf. Consult the Georgia Pest Management Handbook for current pesticide recommendations.
Turf (home lawns)
| Nematode | Assay Result | Recommendation |
| Sting (Belonolaimus longicaudatus) |
20 or more | Turf will likely be affected. |
| Root-knot (Meloidogyne spp.) |
80 or more (primarily bermudagrass) | Turf may be affected. |
| Lance (Hoplolaimus galeatus) |
100 or more | Turf may be affected. |
| Stubby-root (Paratrichodorus spp.) |
100 or more | Turf may be affected. |
| Ring (Criconemella ornata) |
500 or more (150 on centipede) |
Turf may be affected. |
Comments:
The same nematode species that cause problems on golf courses (sting, lance, ring, stubby-root and root-knot) can be pests in home lawns. Unlike golf courses, there are no nematicides that can be used by homeowners or even by lawncare professionals on established home lawns. Therefore, nematode management for home lawns must focus on good turf management practices.
The less stress turf is under, the better it withstands nematode parasitism. Anything done to reduce the overall stress on the lawn will help alleviate pressure from nematodes. Watering the turf deeply and less frequently encourages the growth of deeper roots, which helps minimize nematode problems. Excessive nitrogen fertilizer can lead to a rapid increase in nematode populations as the turf produces succulent roots; proper fertilization of the turf should minimize this problem. Mowing the grass too short increases the stress on the root system and exacerbates nematode problems. Proper disease management on the turf can reduce stress and help reduce nematode damage. Nutrient deficiencies and soil compaction can inhibit root development and increase turf sensitivity to nematode damage. Nematode damage in home lawns is more common in sandy than in heavier soils.
Soil amendments may improve turf quality and overall health, but they must be incorporated before seeding, sprigging or sodding. Any amendment that improves soil composition, moisture-holding characteristics or physical characteristics may be beneficial because it helps produce healthier turf. Amendments that claim to reduce nematode populations may provide a short-term benefit but will not likely provide a lasting solution to nematode problems.
Currently, there are no biological control agents that provide consistent and satisfactory nematode control in turf. If the effectiveness of a biological agent is in doubt, try it on a small patch of turf on a trial basis, but remember to leave an adjacent plot of turf untreated so you will have something to compare the treatment to. Contact your county Extension office for up-to-date information and recommendations.
Status and Revision History
Published on Oct 01, 2001
Published on Feb 10, 2009
Published on Apr 29, 2009
Published with Minor Revisions on Jan 17, 2013


























































