Introduction
Sorghum (Sorghum bicolor) was planted on more than 41,000 acres in Georgia in 2019 with an average yield of 67 bushels per acre and a value of $16.1 million (Table 1).
| County | Acres | Bushels/acre | USD/bushel | Farm gate value (USD) |
|---|---|---|---|---|
| Crisp | 8408 | 71 | 7.96 | 4,761,865 |
| Baker | 8000 | 70 | 3.00 | 1,680,000 |
| Calhoun | 1198 | 80 | 7.96 | 762,886 |
| Randolph | 1291 | 71 | 7.50 | 687,457 |
| Decatur | 2603 | 50 |
5.00 |
650,750 |
| Seminole | 1613 | 65 | 6.00 | 629,070 |
| Jefferson | 1000 | 71 | 7.96 | 565,160 |
| Turner | 1248 | 130 | 3.30 | 535,430 |
| Tift | 680 | 71 | 7.96 | 384,308 |
| Glascock | 850 | 69 | 7.50 | 382,500 |
Sorghum is susceptible to a variety of plant diseases which can adversely affect yield and grain quality. The most common and important diseases in Georgia are discussed below. Terms in bold italics are defined in the text.
Anthracnose
Anthracnose is caused by the fungus Colletotrichum graminicola (syn. C. sublineolum). The disease is prevalent in Georgia, and it is most serious under hot, humid conditions. Anthracnose can infect seedlings and all aerial parts of the plant including leaves, sheaths, stalk, panicle, and seed. The disease can cause grain yield reductions of 50% or more and can cause serious losses to sorghum grown for silage or grazing. C. graminicola produces prolific spores or conidia. The conidia range from hyaline (translucent) to pale brown, are sickle- or spindle-shaped (Figure 1) and are disseminated easily by wind and splashing rain.
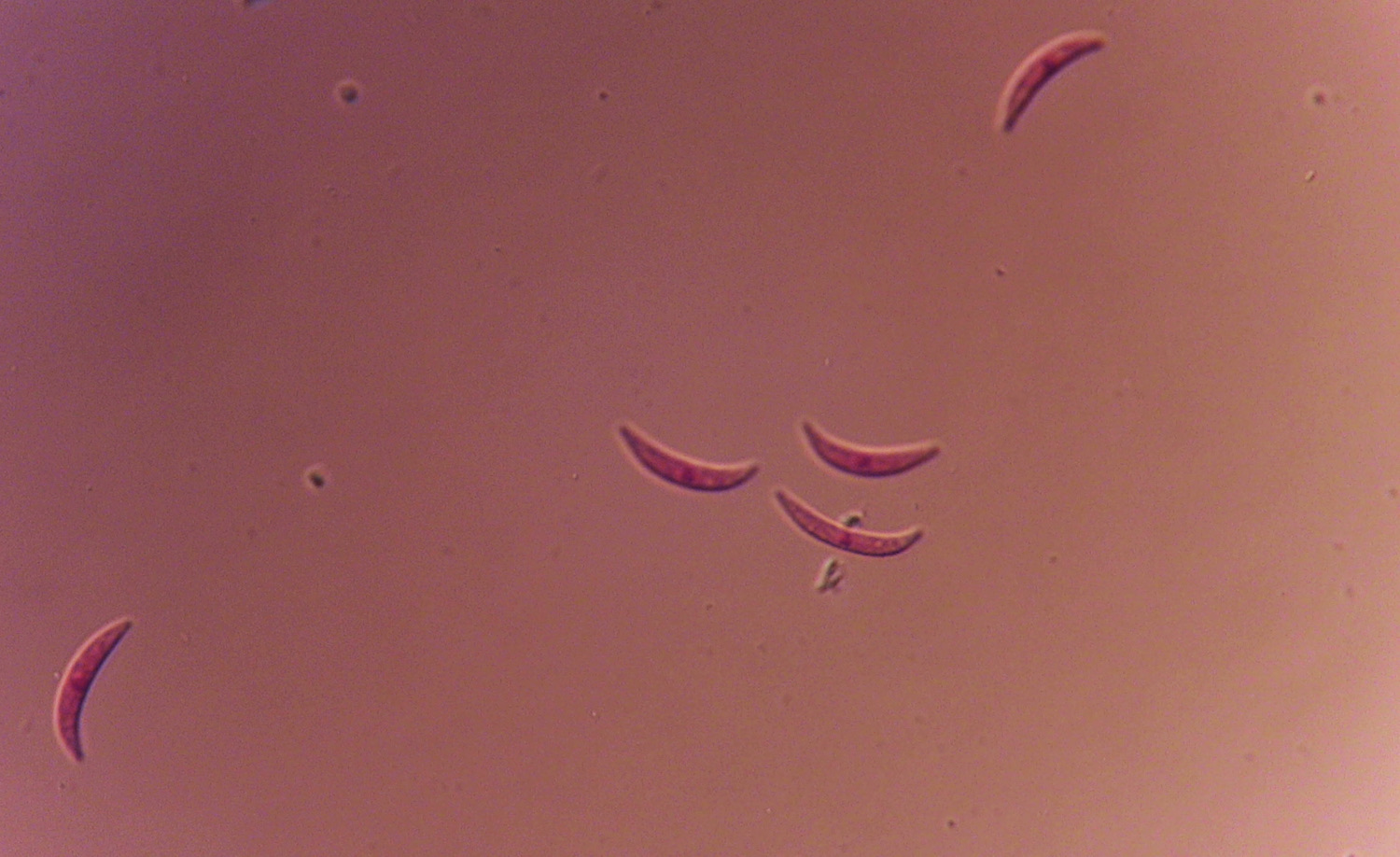
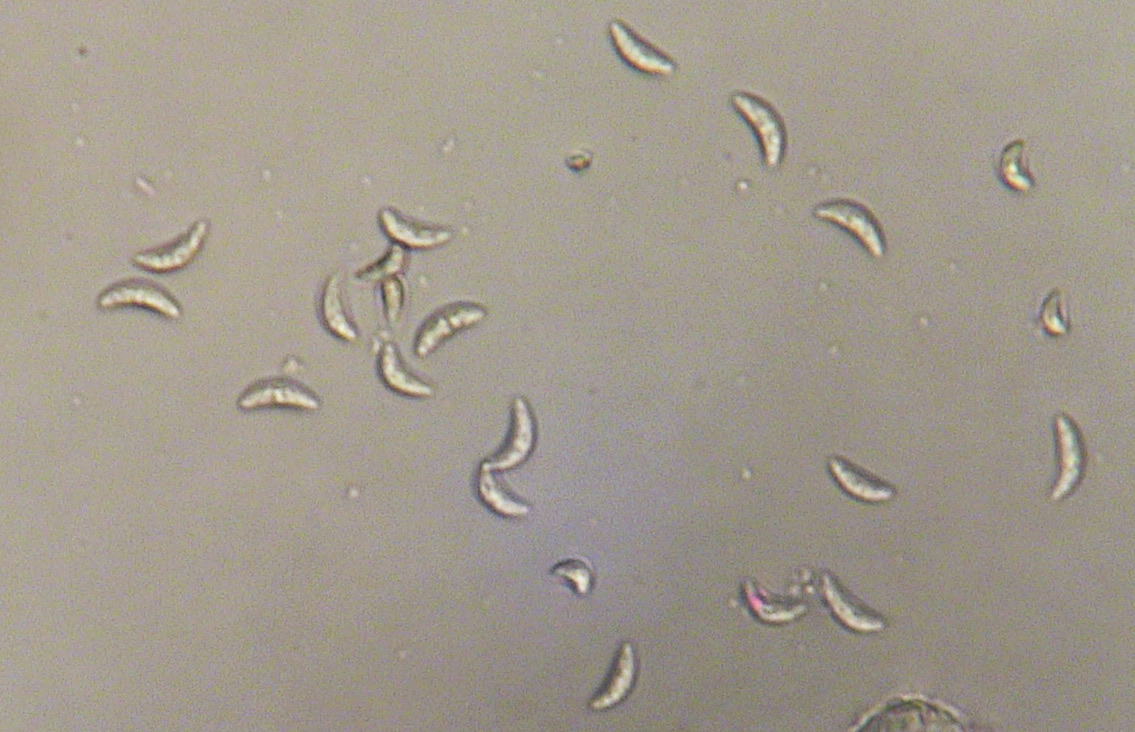
Figure 1. Spores of Colletotrichum graminicola from sorghum. Photo: A. Martinez.
Symptoms of anthracnose include small circular or elongated spots that develop gray or straw-colored centers with wide margins. The margins range in color from red to blackish purple. The symptomatic colors are variable and may change depending on the cultivar and pathogen population. In its severe form, anthracnose can cause premature defoliation. The infection usually extends to leaf sheaths, causing girdling and the collapse of the entire leaf.
The fungus can infect the panicle (head) independently or as result of severe foliar infections. Some or all the florets of the panicle can be affected. In severe cases infection results in broken peduncles (grain stalks). The first symptoms of panicle infections are water-soaked, discolored lesions which later become blackish purple. Severely infected grain is completely discolored, shriveled, small, or nonexistent.
Anthracnose also can affect the stalk, and this appears more common in drier areas, including Georgia. Symptoms include irregularly marbled patterns within the internodal tissue, particularly in the peduncle. Diseased stalks lodge (collapse or break) at the point of infection.
Control
A multiprong approach is best when managing anthracnose. Resistant cultivars are the most economical and environmentally friendly control of anthracnose. Several resistant hybrid cultivars are available commercially in the United States. However, the stability and durability of these cultivars is short-lived because of the development of cultivar-specific virulence in the pathogen and the hypervariability of C. graminicola which allows it to adapt quickly. Crop rotation of at least 2 years with species other than sorghum is advisable.
Elimination of potential weed hosts such as johnsongrass, the elimination of host residues, and properly timed foliar applications of fungicides all can help to control the disease. For a complete and updated list of available fungicide products for sorghum, refer to the latest edition of the Georgia Pest Management Handbook.




Figure 2. Anthracnose symptoms on (A) leaves, (B) whorl, (C) panicle, and (D) grain. Photos: A. Martinez.
Other Foliar Diseases
There are many foliar diseases affecting sorghum in Georgia, some of which may appear at the same time on the same leaf. The most common are gray leaf spot, target leaf spot, leaf blight, and bacterial stripe.
Gray Leaf Spot
This disease is caused by Cercospora sorghi and usually develops at later stages of plant growth, most frequently near crop maturity. Initial symptoms include small red spots on leaves which then enlarge to form narrow rectangular lesions delimited by veins. Lesions coalesce to form blotches, and sporulation (the creation of spores that can later be dispersed) within the lesions give a grayish cast (Figure 3). The pathogen survives on infected host residue (or other infected grasses). Wet, warm conditions are conducive for disease development on infected leaf tissue and the profuse production of spores, which are spread by wind and rainfall.


Figure 3. Gray leaf spot symptoms. Photos: A. Martinez.
Target Leaf Spot
Bipolaris sorghicola is the fungus that causes target leaf spot; it produces reddish to grayish dots which later develop an elliptical or cylindrical shape. Occasionally, the center of the lesion becomes brown or straw colored with purple margins (Figure 4). The fungus can attack during all stages of sorghum development and infections are sustained by high relative humidity and the numerous spores produced on diseased tissue. Target leaf spot may survive between seasons as mycelium (a network of fungal threads), as spores on crop debris, or by actively parasitizing weed hosts such as johnsongrass.


Figure 4. Target leaf spot symptoms. Photos: A. Martinez.
Leaf Blight


Figure 5. Symptoms of a mixed infection: leaf blight, zonate, target leaf spot, and anthracnose. Photos: A. Martinez.
Leaf blight, caused by Exserohilum turcium (syn. Setosphaeria turcica, Bipolaris turcica, and formerly Helminthosporium turcicum), can be problematic if it appears before inflorescence (the formation of the head) and can predispose plants to stalk rot caused by other pathogens. Lesions vary in morphology (form or structure) by cultivar and different levels of host resistance, but generally have yellowish to gray centers with reddish margins (Figure 5).
Sporulation of the pathogen causes necrotic (causing death of localized tissues) lesions with a grayish black appearance, often in concentric zones. Grain usually is not infected. Leaf blight persists as mycelium or conidia in infected crop residue or in the soil. Conidia can be carried long distances by wind and are responsible for secondary spread within and between fields.
Bacterial Diseases
There is anecdotal evidence of bacterial leaf stripe (Burkholderia andropogonis syn. Pseudomonas andropogonis) and bacterial leaf blight (Acidovorax avenae subsp. avenae) in Georgia, but their prevalence and effect on yield is unknown.
Control
The control of the various foliar diseases is like that of anthracnose. Disease management can be achieved by the implementation of sound cultural practices including the use of tolerant or resistant cultivars, rotation with resistant or unsusceptible hosts (other than grasses), and elimination of weed hosts such as johnsongrass. Hastening the decomposition of old crop residue also helps to control the spread of these diseases.
Foliar fungicides may be available for specific diseases in certain areas. For a complete and updated list of available fungicide products refer to the latest edition of the Georgia Pest Management Handbook.
Fusarium Stalk Rot


Figure 6. Stalk rot symptoms in corn, which are similar to those observed in sorghum. Photos: Alison Robertson, Bugwood.org.
Stalk rot is caused by Fusarium thapsinum, and can affect many grasses, including grain sorghum. Yield reductions often are attributed to poor grain filling, lodged peduncles, and lodging. Stalk rot can cause root damage, resulting in the loss of anchorage (the roots’ grip on the soil) as well as reduced water uptake.
Symptoms vary depending on cultivar genotype, pathogen genetic makeup, and environmental conditions, and they may appear at any time during the growing season. Lesions vary from small reddish spots to purple elongated streaks and can appear on internal or external tissues of stalks, peduncles, roots, or seeds. Fusarium infections typically display areas of reddish-tinted vascular bundles (Figure 6).
Initial infections can originate from mycelium or conidia that have survived from previous seasons in crop debris, or from infested seeds. Soilborne or airborne inoculum (e.g., spores, mycelium) can contribute to initial infections. The fungus takes advantage of natural openings or injuries to roots and stalks. Plant stress, as well as cool and wet conditions followed by hot and dry weather, are conducive to disease development.
Control
Fusarium stalk rot can be greatly reduced by implementing conventional tillage systems, selection of varieties resistant to lodging, providing full soil moisture, reducing plant populations, implementing weed control, providing balanced soil fertility, and implementing insect control.
Grain Mold
This disease is caused by several fungi including Fusarium spp. (F. thapsinum, F. semitectum, others), Curvularia lunata, Alternaria sp., Bipolaris/Drechslera spp., and Phoma sp. Grain mold is more likely to occur when high moisture conditions are present near harvest time and when harvest is delayed.


Figure 7. Symptoms of a mixed infection of grain mold. Figure 7B shows grain mold initiated by bird feeding. Photos: A. Martinez.
Generally, sorghums with dense, compact heads are more prone to attack than are varieties with loose, open heads. The appearance of symptoms depends upon the predominant fungus and severity of the infection. In some cases, severely infected panicles show grains fully covered with mold, but also may appear as normal-looking grains with only a slight discoloration (Figure 7). The molded grains may have pink, orange, gray, white, or black discoloration. Heavily infected grains can disintegrate under the slightest pressure, while grains infected early in the season often are aborted or have reduced kernel size and weight.
Control
One successful management strategy is growing cultivars that do not mature during seasonal rains, or those that ripen during dry weather after rains have stopped. Grain mold can be minimized by growing high-tannin colored-grain cultivars. However, the most common and economical control for grain mold is the use of resistant cultivars. Weed control is important because weeds compete for water and nutrients. Insect control is important because insects contribute to plant stress and wound formation. Reduce stressful competition for moisture and nutrients by maintaining balanced soil fertility to ensure sturdy plants and avoid excessive plant populations.
A 2-year rotation with plant species other than sorghum will help to minimize the amount of inoculum in the field. Clean cultivation, elimination of probable weed hosts (e.g., johnsongrass), and encouraging rapid decomposition of host residue also help to control the disease. While there are fungicides labeled for control of grain mold, they are difficult to implement and usually not economical. For a complete and updated list of available fungicide products, refer to the latest edition of the Georgia Pest Management Handbook.
Ergot
Recently, more samples submitted to the UGA Plant Disease Clinic have been diagnosed as ergot. Ergot is caused by the fungus Claviceps africana and may result in significant grain loss from lower yields and reduced grain quality.


Figure 8. Symptoms of ergot. Notice the honeydew on the panicle. Photos: A. Martinez and R. Ethredge.
One striking and unique symptom of ergot is the production of honeydew from the infected florets (Figure 8), which can vary in color from whiteish tan to brown. The honeydew’s viscosity also is variable; it may be thin or viscous and its viscosity does not correspond to its color. Within the honeydew are conidia that can be spread easily by mechanical contact, wind, rain, or insects. Parasitic bodies of the fungus may develop instead of seed, or they may produce sclerotia, which are hard, compact masses of mycelium that help the ergot survive unfavorable growing conditions.
Florets are vulnerable to fungal infection during the period from floret gaping and stigma exertion to pollination or fertilization of the ovary, especially under wet, cloudy weather. Fungal infection decreases with increasing temperature and is negligible at temperatures higher than 86 °F (30 °C).
Control
The flower-infecting nature of the disease makes it extremely difficult if not impossible to implement scouting activities. Therefore, the disease can be managed by integrating several cultural and chemical strategies.
Adjustments in sowing dates and locations can help to avoid ergot infections. The use of clean, treated seed can help reduce the amount of sclerotia present on infested seed and reduce initial inoculum. Clean cultivation, elimination of weed hosts (e.g., johnsongrass) and host residue, as well as the prevention of volunteer plants or sorghum ratoon (regrowth of plants cut for harvest) can help to control the disease. Harvesting prior to heading (when the panicle becomes visible) is recommended for sorghum destined for forage.
Although chemical control is difficult to implement, there are fungicides labeled for ergot control. For a complete and updated list of available fungicide products, refer to the latest edition of the Georgia Pest Management Handbook.
Other Diseases
In Georgia we have observed sporadic infections of bacteria (e.g., bacterial stripe, bacterial streak), viral diseases such as maize dwarf mosaic virus (MDMV), and nematodes (stunt, root-knot, and lesion). However, their prevalence and economic damage in Georgia is unclear.
References
Bandyopadhyay, R., Frederickson, D., McLaren, N. W., Odvody, G. N., & Ryley, M. J. (2007). Ergot: A new disease threat to sorghum in the Americas and Australia. The American Phytopathological Society. https://doi.org/10.1094/PDIS.1998.82.4.356
Frederiksen, R. A., & Odvody, G. N. (2000). Compendium of sorghum diseases. American Phytopathological Services Press. https://my.apsnet.org/APSStore/Product-Detail.aspx?WebsiteKey=2661527A-8D44-496C-A730-8CFEB6239BE7&iProductCode=42406
Jardine, D. (2006). Stalk rots of corn and sorghum. Kansas State University Agricultural Research Station and Cooperative Extension Service. https://www.plantpath.k-state.edu/extension/publications/field-crops/corn-sorghum-stalk-rot-L741.pdf
Krausz, J., & Isakeit, T. (1998). Sorghum ergot: New disease threat to the sorghum industry. Texas A&M Agrilife. https://agrilifelearn.tamu.edu/s/product/sorghum-ergot-new-disease-threat-to-the-sorghum-industry/01t4x000004Ofrc
Wrather, A., & Sweets, L. (2022). Management of grain sorghum diseases in Missouri. University of Missouri Extension. https://extension.missouri.edu/publications/g4356
Status and Revision History
Published on Jul 12, 2023
