Oats (Avena sativa) are an important multipurpose crop in Georgia: they are produced for grain and forage, as a nitrogen catch-crop, as a cover crop for erosion control, and as green manure to enhance soil health. In 2019, approximately 29,000 acres of oats were planted for grain in Georgia with an average yield of 57 bushels per acre and a value of $6.7 million (Table 1).
| County | Acres | Bushels / acre | Price / bushel | Farm gate value |
|---|---|---|---|---|
| Decatur | 2,403 | 55 | 4.10 | $541,876 |
| Telfair | 2,136 | 55 | 4.10 | $481,668 |
| Dodge | 1,800 | 55 | 4.50 | $445,500 |
| Laurens | 1,789 | 55 | 4.10 | $403,437 |
| Burke | 1,412 | 55 | 4.10 | $329,681 |
| Early | 831 | 75 | 4.10 | $225,532 |
| Mitchell | 995 | 55 | 4.10 | $224,372 |
| Johnson | 900 | 60 | 4.00 | $216,00 |
| Brooks | 933 | 55 | 4.10 | $210,391 |
| Wheeler | 900 | 55 | 4.10 | $202,950 |
Oats are susceptible to a variety of plant diseases that can adversely affect yield and grain quality; if not managed properly, these diseases can cause complete field loss. The most common and important diseases in Georgia are discussed below.
Crown/Leaf Rust

Photo A: Louisiana State University AgCenter, Bugwood.org
Photo B: A. Martinez
Crown/leaf rust, caused by the fungal pathogen Puccinia coronata f. sp. avenae, is one of the most damaging oats diseases in Georgia. Yield losses associated with crown/leaf rust range from slight or moderate to full crop failure.
Crown rust symptoms include small, flaky, round pustules that erupt through the upper and lower leaf surfaces. The pustules are yellow to red and produce spores of the same color (Figure 1).
Spores can be spread by wind, rain, and contact with neighboring foliage. Under moist environmental conditions such as dew, rain, and increased humidity, spores can germinate rapidly, infect adjacent leaves or plants, and produce new pustules within 7–10 days. The fungus overwinters on volunteer oats and wild oats. Alternate hosts of this pathogen are species within the genus Rhamnus, commonly known as buckthorns. Crown rust does not affect wheat, barley, or rye.
Control: The use of resistant cultivars is one of the most effective and economical ways to reduce losses caused by rusts. Table 2 lists the relative rust-resistance of oat varieties. However, changes in the genetic makeup of pathogens may render resistant cultivars ineffective over time. Visit the statewide variety testing website (swvt.uga.edu) and look for the most recent Winter Grains & Forages report to find up-to-date oat cultivar recommendations.
| Variety | Crown Rust | Stem Rust |
|---|---|---|
| Big Mac | MS | S |
| Bob | S | MS |
| Coker 234 | MS | S |
| Coronado | MS | MR |
| Florida 501 | S | MS |
| H-422 | MR | S |
| H-833 | MS | S |
| Mesquite | MS | S |
| Nora | S | S |
Properly timed foliar applications of fungicides—specifically the classes of demethylation inhibitors (DMI), quinone outside inhibitors (QoI), and succinate dehydrogenase inhibitors (SDHI)—can protect against crown rust. Products should be applied preventatively during flag-leaf emergence to inhibit infection. Once the pathogen has infected the plant, fungicides will no longer be an effective control. For a complete and updated list of available fungicide products, refer to the latest commercial edition of the Georgia Pest Management Handbook (ipm.uga.edu/georgia-pest-management-handbook/).
Leaf Blotch, Victoria Blight, and Culm Rot
Leaf blotch, caused by Drechslera avenacea, creates oblong linear blotches that start off light-yellow in color and turn red to brown on the leaf tissue as they mature (Figure 2). Victoria blight, caused by Bipolaris victoriae, is most commonly observed as seedling disease, resulting in plant collapse after emergence. Culm rot, caused by Bipolaris maydis, causes the stem and roots to rot and eventually kills the plants.
All three of these fungi can cause economically significant damage to oats. In Georgia, leaf blotch is the most common disease of the three.
Control: Management of these diseases is dependent on reducing the pathogen’s ability to infect plants through cultural practices such as tillage, crop rotation, and destruction of volunteer oats. Seed treatments also can be an effective management option. In Georgia, leaf blotch can infect early in the season, but the disease tends to diminish as temperatures rise and it rarely requires a fungicide application.

Photo: A. Martinez
Smuts
Loose smut, caused by Ustilago avenae, and covered smut, caused by Ustilago kolleri, are the two most common smut diseases in Georgia. The symptoms and signs of smut diseases commonly occur within the oat panicle. Black to dark-brown balls of loosely attached smut spore masses typically replace the oat kernels, but not the outer hulls (Figure 3). Symptoms and signs of smuts are evident at heading, once the seed heads have emerged from the stalks. Smut spore masses can be spread by wind and rain, remaining intact inside the hulls until harvest.
Control: Because U. avenae and U. kolleri live systemically within the plant throughout the growing season, the use of certified disease-free seed is recommended to reduce disease incidence. For a complete and updated list of available fungicide products, refer to the Georgia Pest Management Handbook.

Photos: A. Martinez
Fusarium Head Blight
Fusarium head blight (FHB) is caused by several fungal species in the genus Fusarium. Bleached florets in the spike or head are the best identifiers of FHB. Other identifiers include tan or brown discoloration at the base of the head. Under moist conditions, there usually is a pinkish/orange mycelium at the base of the florets, and kernels that are shriveled, white, and chalky in appearance—resembling tombstones (Figure 4). Perithecia, the dark fruiting bodies in fungi, are produced within the mycelium later in the infection process. Diseased, bleached spikelets are sterile or contain shriveled/discolored seed, usually with a tint of pink or orange. Severe infections can cause premature blight or bleaching of the entire spike.
Control: Fusarium head blight is difficult to control, so it’s best to manage it through a multipronged approach. FHB forecasting models, such as the online Fusarium Risk Tool (www.wheatscab.psu.edu), can be helpful, but constant monitoring of the planting site still is needed as sometimes the models either exaggerate or downplay the probability of infection. Forecasting is county-dependent or even field-dependent, and several factors must be taken into consideration, such as the variety of oats and whether flowering times coincide with wet conditions and moderate temperatures. For more information on forecasting, see the website for the U.S. Wheat and Barley Scab Initiative (scabusa.org).
Crop sequence, or what crops were planted and when, and tillage, the incorporation of crop residues into the soil, have been shown to affect the incidence of Fusarium head blight. Sorghum and corn are host for some Fusarium species that cause FHB. In recent years, decreases in tillage may have contributed to the increase in regional FHB epidemics by increasing the levels of the pathogen that are available to cause infection. Managing the residue of cereal crops like corn and sorghum will reduce the amount of overwintering inoculum, or pathogen sources, that can infect a subsequent oat crop.
The relative contribution of inoculum from local and distant sources is not fully understood. Local management of the disease may not be effective in regions where there is a significant source of airborne inoculum. Losses may be avoided by using staggered planting and/or planting varieties with different maturities that flower at different times. If possible, avoid irrigation during flowering to reduce humidity and therefore reduce the infection period.
Control of Fusarium head blight using fungicides can be difficult because they need to be applied at a specific time and the selection of fungicides labeled for FHB is limited. Foliar sprays with a triazole fungicide work best when applied at early flowering or within a week. The use of nozzles that provide good coverage of the spike is essential for proper disease management. Strobilurin fungicides are not recommended because data from other states seems to indicate that these fungicides can increase the deoxynivalenol (DON) mycotoxin content of FHB-infected grain.
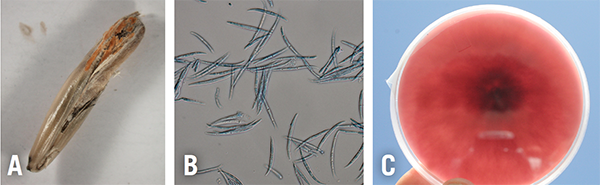
Figure 4B. Spores of Fusarium spp. recovered from infected oats.
Figure 4C. Isolate of Fusarium spp. growing in culture.
Photos A and B: Cesar Calderon, Cesar Calderon Pathology Collection, USDA APHIS PPQ, Bugwood.org.
Photo C: A. Martinez
Barley Yellow Dwarf Virus
Barley yellow dwarf virus (BYDV) is caused by two viral pathogens belonging to Luteovirus and Polerovirus. These viruses are carried from plant to plant by over 25 different species of aphids. Plants infected with BYDV display symptoms such as stunted growth and reduced root mass, which may result in reduced head emergence. Research data has shown these pathogens can reduce yields by up to 80%. Dwarfed plants range in color from red, orange, or yellow to purple (Figure 5). Symptoms of BYDV usually appear in conjunction with infestations of aphids or smallgrain greenbugs.
Control: The management of barley yellow dwarf virus is dependent on control of aphids. The use of insecticidal seed treatments as well as timely foliar applications of insecticides can be effective in managing BYDV vector population levels.

References
Arceneaux, K., Beuzelin, J., Buckley, B., Griffin, J., Groth, D. E., Harrison, S., Hollier, C., Huang, F., Kerns, D. L., Levy, R. J., Jr., Lofton, J., Mascagni, H. J., Jr., Miller, K., Padget, B., Price, T., Purvis, M., & Stephenson, D. (2014). Wheat and oat weed, insect and disease field guide (Publication No. 3306). Louisiana State University Ag Center. https://www.lsuagcenter.com/portals/communications/publications/publications_catalog/crops_livestock/grain%20crops/wheat-and-oat-weed-insect-and-disease-field-guide
Buntin, G. D., & Cunfer, B. M. (2017).Southern small grain resource management handbook (Bulletin 1190). University of Georgia Cooperative Extension. https://extension.uga.edu/publications/detail.html?number=B1190&title=Southern%20Small%20Grains%20Resource%20Management%20Handbook
Taylor, M., Sial, A., & Cabrera, E. (Eds.). (2022).Georgia pest management handbook: Commercial edition(Special Bulletin 28). University of Georgia Cooperative Extension.https://extension.uga.edu/publications/detail.html?number=SB28
Texas A&M Agrilife Extension. (n.d.) Texas plant diseases handbookhttps://plantdiseasehandbook.tamu.edu/food-crops/cereal-crops/oats/
University of Georgia Center for Agribusiness and Economic Development. (2020, December).Georgia farm gate value report 2019.https://caed.uga.edu/content/dam/caes-subsite/caed/publications/annual-reports-farm-gate-value-reports/2019%20Farm%20Gate%20Report.pdf
University of Georgia College of Agricultural and Environmental Sciences. (2022).Statewide variety testing.https://swvt.uga.edu
Wegulo, S. N., & Hein, G. L. (2013).Yellow dwarf wheat, barley, and oats (Publication No. G1823). University of Nebraska-Lincoln Extension.https://extensionpublications.unl.edu/assets/pdf/g1823.pdf
Status and Revision History
Published on May 03, 2023
