In the equine industry, parasite control is primarily accomplished using anthelmintics (dewormers). Still commonly used, the previous recommendation for treating parasites was a rotating program of dewormers on an approximate 8-week schedule.
Many horse owners implement this outdated recommendation today without realizing that newer recommendations have been released by the American Association of Equine Practitioners (AAEP) due to increasing incidence of parasite resistance to dewormers (AAEP Parasite Control Subcommittee, 2019).
The Evolution of Equine Parasites
In the 1960s, rotational deworming practices gained popularity when a significant reduction in the most prevalent parasite, large stronglyes (Strongylus vulgaris), was noted in response to deworming treatments.
Due to the widespread use of dewormers, the large strongyle population was virtually eradicated. Today, the most predominant parasitic threat to mature horses is small stronglyes (cyanthostomins). Figure 1 outlines the life cycle and transmission of small strongyles in horses.
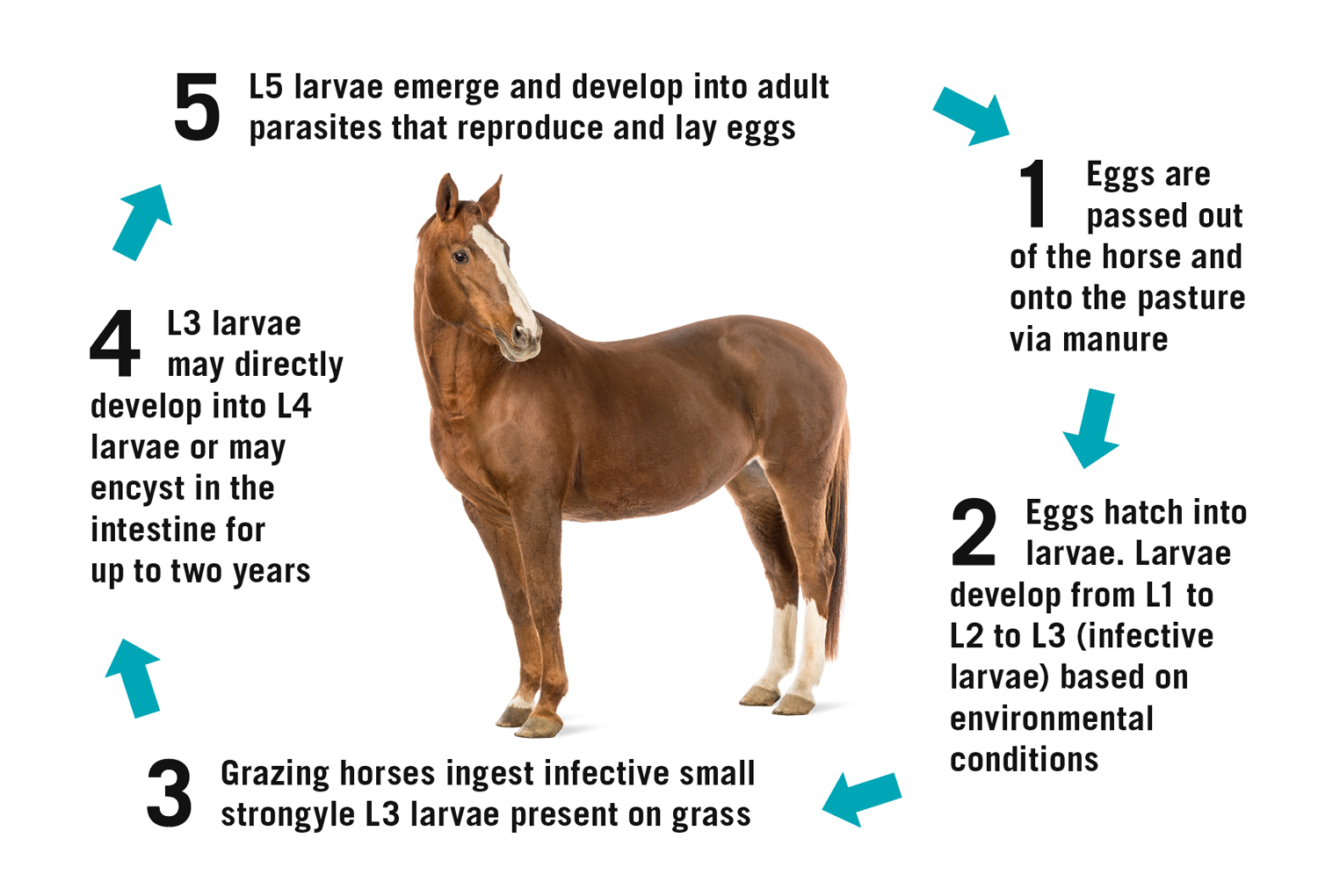
Cyathostomins have a unique life cycle. Eggs are passed from adult worms through the feces to begin their development in the pasture. Larval stages one through three take place in the pasture and their rate of development is highly dependent on climate; ambient temperatures and adequate moisture correlate to faster maturation to the third larval stage.
Once this infective third stage is reached, the larvae become encased in protective membranes, which equips them to withstand freezing temperatures and remain in the pasture for longer periods of time. The larvae are ingested by the horse, allowing for the removal of the protective sheath as they enter the mucosa of the large intestine. The third-stage larvae have the distinct ability to encyst themselves in the intestinal wall for further protection. They can remain here for up to 2 years before they emerge and continue developing to their fourth and fifth stages, eventually reaching maturity as an adult parasite in the cecum or colon. At this stage, they lay eggs to be passed through the feces as the next generation of small strongyles begin (Corning, 2009).
Small stronglyes play a role in an array of health problems for horses, although they produce milder disease conditions compared to large strongyles. Similar to other parasites, if present in high enough levels in the gut, they can cause lethargy, weight loss, and debilitation. However, their special ability to encyst in the gut wall allows for a multitude of other problems regarding parasite management in horses. The encysted larvae can number in the millions, yet the animal can read as a low shedder on a fecal egg count test.
A 1999 study found that if there is a sudden, substantial reemergence of encysted parasites, numbering the several millions, larval cyathostominosis occurs. This inflammation of the intestines can have profound pathological effects, such as sudden weight loss, edema, diarrhea, and even potential death (Love et al., 1999). An additional study in 2002 reported that the encysted larvae can number high enough to cover a great majority of the gut wall, resulting in damage and inhibiting nutritional uptake (Collobert-Laugier, 2002).
From a treatment and management standpoint, small strongyles’ unique ability to encyst themselves in the gut wall until conditions are favorable for their survival makes them problematic. This distinctive trait has led to the development of small strongyle resistance to rotational deworming because most dewormers are only able to kill the parasites in the lumen of the intestine (e.g., not the encysted strongyles).This development has resulted in the American Association of Equine Practitioners (AAEP) no longer recommending an 8-week rotational deworming program.
Types of Chemical Dewormers
There are currently three main classes of dewormers:
- benzimidazoles (fenbendazole, oxibendazole)
- tetrahydropyrimidines (pyrantel)
- macrocyclic lactones (ivermectin, moxidectin)
The World Association for the Advancement of Veterinary Parasitology considers resistance to occur if there is a 95% or less effective rate of the drug to the target parasite load (meaning that less than 95% are killed after treatment; Coles et al., 2006). Resistance is an inherited trait that can be passed from one generation of parasite to the next. The great population size in conjunction with high levels of genetic diversity has allowed small stronglyes to rapidly develop resistance to dewormers.
For example, the high genetic diversity of these parasites may allow for a select number of them to be naturally resistant to a drug. Once the drug treatment is administered, the naturally resistant worms have survived, mated with each other and passed their genome to the next generation, which is then born with the genetics for resistance. When several doses of drug treatment have been administered, the number of resistant worms continues to rise, because the drug only wipes out those worms that are not able to withstand treatment.
Resistance to dewormers is assessed using a fecal egg count reduction test (FECRT) in which fecal egg counts are assessed before treatment (deworming) and again at 14 days after treatment. Resistance would be defined as the failure of the drug to have a high fecal egg count reduction in this 14-day period.
In a 2004 study, it was shown that the percentage of farms in the Southern U.S. harboring resistant small strongyles was 97.7% for fenbendazole, 53.5% for oxibendazole, and 40.5% for pyrantel pamoate (Kaplan et al., 2004). In this study, 0% of farms harbored small strongyles that were resistant to ivermectin. However, the effectiveness of a deworming agent can also be measured by the egg reappearance time (ERP), which assesses the length of time after deworming before eggs are present again in the fecal samples of a treated horse. The ERP for small strongyles after treatment with ivermectin or moxidectin has been decreasing, meaning that parasites are coming back faster than they did when the drugs were first marketed (Lyons et al., 2008).
For all of these reasons, the current recommendations for deworming programs are much different from the rotational deworming program that was implemented 40+ years ago. In spite of all of the recent compelling evidence to move away from regular rotational deworming, many horse owners still adhere to this outdated method. If this trend continues, the equine industry may be faced with a similar problem that sheep and goat owners have today—high parasite resistance to all classes of dewormers with almost no effective treatment to combat parasitic infections.
New Parasite Control Recommendations
So what are the current deworming recommendations and how can horse owners help in preventing continued resistance problems?
- Move away from rotational deworming. It has been shown that rotating classes of dewormers does not result in less appearance of parasite resistance. Since small strongyles show significant resistance to two of the three main classes of dewormers, rotating anthelmintics is not recommended. Ivermectin (or moxidectin) should be the basis of a deworming program for adult horses. In many cases, one to two deworming treatments per year is sufficient to control large strongyles and to treat for other parasites such as tapeworms and bots (treating tapeworms requires the use of praziquantel, which is included only in some ivermectin/moxidectin dewormers).
- Perform fecal egg tests to develop a deworming plan. An individual horse owner can learn to perform these if they have access to the correct tools (10X microscope, slides, fecal flotation solutions). Alternatively, a licensed veterinarian can perform these when doing spring and fall vaccinations. It is important to collect fecal samples at an appropriate time after administering a dewormer. If fecal samples are collected and analyzed too soon after deworming, a low fecal egg count is more likely to be representative of the action of the dewormer rather than the horse’s innate egg shedding characteristics. Appropriate timing when collecting fecal samples varies based on the dewormer used. The AAEP recommends waiting at least 16 weeks following administration with moxidectin, at least 12 weeks for ivermectin, and at least 9 weeks following benzamidazoles (AAEP Parasite Control Subcommittee, 2019).
- Deworming treatments for all horses should occur when parasite loads are highest, typically in the fall and spring in the Southeastern U.S., as extreme heat or cold reduces pasture levels of infective parasites. Any treatment beyond this should be done based on the small strongyle egg shed rate (determined by the fecal egg test) of the individual horse. This means that rather than deworming all horses beyond once or twice a year, collect individual fecal samples and count the eggs so that only those horses with moderate to high fecal egg counts are dewormed.
Horses are typically classified as low, moderate, or high egg shedders based on the amount of small strongyle eggs per gram (EPG) present in their feces, with Table 1 showing a scale that is commonly used to classify horses. Horses typically have fairly consistent egg shed rates, regardless of parasite treatment, as long as their health and stress levels remain the same. What this means is that high shedders will likely always be high shedders, even when dewormed at more frequent intervals, while low shedders will likely remain as low shedders for most of their lives. It is important to understand that horses that are higher shedders don’t necessarily have higher populations of internal parasites. The differences in age and immune status of each horse influences the number of eggs they shed. However, moderate and high egg shedders result in greater transmission of infective parasite larvae in pastures and therefore are the target of selective deworming programs at more regular intervals throughout the year. Some low shedding horses may only need to be dewormed once a year to maintain their health, whereas high shedders may need to be dewormed multiple times throughout the year to reduce pasture transmission of parasites. In most cases, you cannot visually distinguish a high shedder from a low shedder, so performing fecal egg counts is important. Working with your veterinarian to develop a selective deworming plan is the best way to combat parasite resistance on your farm. - Deworm based on the life stage of your horse. Young horses (particularly foals) are more prone to ascarids (small intestinal roundworms) than older horses. Due to the fact that ascarids show high resistance to ivermectin, deworming programs for foals, particularly prior to weaning, should implement the use of benzimidazoles starting at about 2 to 3 months of age and again around 4 to 6 months of age. Following weaning, a fecal egg count can help assess whether ascarids or strongyles are more prevalent in the individual horse and guide which anthelmintic is the best choice. Since younger horses are considered more susceptible to parasites, yearlings and 2-year-olds should be classified as high shedders and dewormed more frequently with the appropriate dewormer based on the most prevalent parasite quantified in fecal egg tests.
- Implement other nonchemical methods of parasite control in addition to deworming. Removing manure from pastures can be an extremely effective way to control parasite transmission if manure is removed quickly (multiple times per week). Properly composting manure before it is applied to pastures also significantly reduces pasture transmission because the heat from the composting process will kill parasite eggs. Uncomposted manure should not be spread on pastures. Rotational grazing and grazing with other ruminant species may be beneficial in some areas, depending on the climate.
| Low shedders | Medium shedders | High shedders | |
|---|---|---|---|
| Fecal egg count* | < 200 epg | 200–500 epg | > 500 epg |
| Deworming recommendation** | One to two times per year with ivermectin/moxidectin and praziquantel (tapeworms) | One to two times per year with ivermectin/moxidectin and praziquantel Additional ivermectin/moxidectin treatments recommended based on FEC |
One to two times per year with ivermectin/moxidectin and praziquantel Additional ivermectin/moxidectin treatments recommended based on FEC |
| *Fecal egg count reported as eggs per gram of feces (epg). **Deworming recommendations are based on general recommendations. Owners are encouraged to work with their veterinarian to develop a deworming program specific to their individual horses. |
|||
Summary
Recommendations for parasite control have changed in recent years, but the equine industry as a whole has been slow to implement current recommendations. It is important to recognize that the goal of a deworming program is to maintain the health of the entire herd of horses rather than eliminating the parasite population in a single horse. All mature horses need to be dewormed with an appropriate dewormer once to twice per year to maintain their individual health.
Beyond that, deworming should be selectively applied, keeping in mind that the goal of the deworming program is to reduce egg transmission to pastures from higher shedding horses. Implementing good pasture management practices while working with a veterinarian to develop individualized deworming plans will go a long way in combating parasite resistance so that dewormers remain effective for years to come.
For more information on parasite control in horses, contact your local Extension office or call 1-800-ASK-UGA-1.
References
AAEP Parasite Control Subcommittee. (2019). AAEP Parasite Control Guidelines. AAEP Infectious Disease Committee. https://aaep.org/sites/default/files/Guidelines/AAEPParasiteControlGuidelines_0.pdf
Coles, G., Jackson, F., Pomroy, W., Prichard, R., von Samson-Himmelstjerna, G., Silvestre, A., Taylor, M. A., & Vercruysse, J. (2006). The detection of anthelmintic resistance in nematodes of veterinary importance. Veterinary Parasitology, 136(3–4), 167–185. https://doi.org/10.1016/j.vetpar.2005.11.019
Collobert-Laugier, C., Hoste, H., Sevin, C., & Dorchies, P. (2002). Prevalence, abundance and site distribution of equine small strongyles in Normandy, France. Veterinary Parasitology, 110(1–2), 77–83. https://doi.org/10.1016/S0304-4017(02)00328-X
Corning, S. (2009). Equine cyathostomins: a review of biology, clinical significance and therapy. Parasites & Vectors, 2(Suppl 2). https://doi.org/10.1186/1756-3305-2-S2-S1
Kaplan, R. M., Klei, T. R., Lyons, E. T., Lester, G., Courtney, C. H., French, D. D., Tolliver, S. C., Vidyashankar, A. N., & Zhao, Y. (2004). Prevalence of anthelmintic resistant cyathostomes on horse farms. Journal of the American Veterinary Medical Association, 225(6), 903–910. https://doi.org/10.2460/javma.2004.225.903
Love, S., Murphy, D., & Mellor, D. (1999). Pathogenicity of cyathostome infection. Veterinary Parasitology, 85(2–3), 113–122. https://doi.org/10.1016/S0304-4017(99)00092-8
Lyons, E. T., Tolliver, S. C., Ionita, M., Lewellen, A., & Collins, S. S. (2008). Field studies indicating reduced activity of ivermectin on small strongyles in horses on a farm in Central Kentucky. Parasitology Research, 103(1), 209–215. https://doi.org/10.1007/s00436-008-0959-7
Status and Revision History
Published on Mar 27, 2020
Published with Full Review on Jun 09, 2023


























































