
As the cultivation of blueberries has expanded and intensified in Georgia, several new, economically important disease problems have emerged, a recent example of which is Exobasidium leaf and fruit spot (Figure 1). This early-season disease affects several cultivars of rabbiteye (Vaccinium virgatum) and southern highbush (Vaccinium corymbosum interspecific hybrid) species, causing spots on susceptible young, tender leaves, shoots, and fruit. In the Southeastern region of the United States, the disease was first described in North Carolina (1998). In Georgia, the first sporadic occurrence of Exobasidium leaf and fruit spot was noted in 2010. Although initially not of major concern, subsequent growing seasons resulted in reports of marked increases in both disease prevalence and intensity, leading to widespread losses due to the downgrading or rejection of affected fruit at the packing house. The disease is caused by the plant-pathogenic fungus Exobasidium maculosum, a new species described in 2014.
SYMPTOMS

Beginning in early spring (late March to early April), small (1 to 3 millimeter), slightly yellow spots develop on
the upper leaf surface of newly expanding leaves (Figures 1, 2, and 3A-D). On the lower leaf surface, the spots are initially light green to white (Figure 3B). Over the course of one to two weeks, the spots expand, reaching a diameter of 2 to 10 millimeters. Prior to sporulation, or the formation of spores, mature leaf spots have a characteristic light yellow-green halo surrounding a yellow center (Figure 3C) and are primarily visible on the lower surface of the leaf. As spots develop further, the halo disappears and the center turns yellow-green. Once mature, these spots lighten, becoming increasingly white (Figure 3D) as the fungus sporulates on the underside of leaves and on the surface of infected fruit and shoots. One of the diagnostic characteristics of the disease is the velvety, white appearance on the underside of sporulating leaf spots. Postsporulation, spots gradually become brown and necrotic with a distinct reddish-brown margin (Figure 3E). Leaf spots that occur on or near petioles often result in defoliation. Bushes with a high disease severity experience significant defoliation, whereas blueberry bushes with leaves containing only one or two spots do not defoliate as frequently. Defoliation is generally more pronounced within the lower half of the bush.

Characteristic Exobasidium fruit spots are easily recognized on ripe fruit, but with careful observation, symptoms may also be found on young, green fruit, 0.25 to 1.0 centimeters in diameter. It is important to note that the light- green symptoms of fruit spot that present on green fruit are usually only observable by removing the surface wax layer (Figure 4A). As affected fruit begin to ripen and darken, the immature light-green spots become increasingly obvious (Figure 4B and C). When spots reach maturity, they often appear slightly white to gray due to sporulation on the fruit surface. As fruit ripen, fruit spots become increasingly conspicuous and are noted as having a distinctly green or magenta appearance (Figure 4C). Postsporulation, fruit spots become necrotic, which may enable secondary pathogens to colonize. There is also strong evidence to suggest that severe infection may cause premature fruit drop. While the exact level of premature fruit drop is not known, one study estimated that the incidence of in-bush infected fruit decreased by over 50% due to premature fruit drop prior to harvest. Infected fruit are also generally smaller than noninfected fruit.

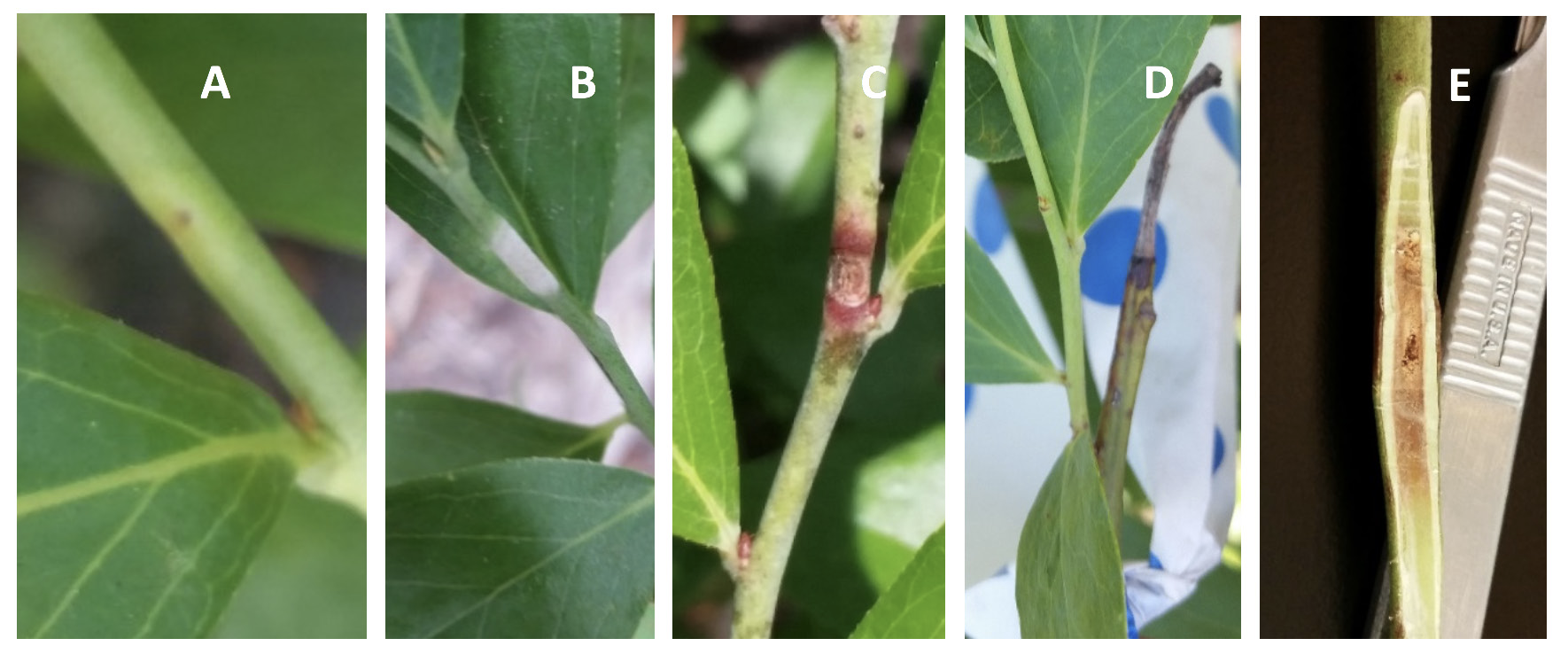
While less prevalent than the leaf and fruit spot symptoms, spots also form on young, actively growing shoots early in the season. These shoot spots cause damage to new shoots due to the progressive necrosis of tissue that often results in girdling of the infected shoot. Shoot spots first appear as slightly chlorotic lesions on one side of young, tender current-season shoots, containing a small dark point in the center, along with or without a darkened margin (Figure 5A). As shoot spots develop further and expand, infected shoot tissue becomes increasingly white and raised. At maturity, shoot spots appear white and velvety due to the sporulation of the fungus (Figure 5B). Postsporulation, shoot spots become brown to black and necrotic with a distinctive reddish-brown margin (Figure 5C). Shoot spots then form a canker that in some cases completely girdles the shoot (Figure 5D), meaning that the canker forms a ring around the shoot in which the top layers of tissue have atrophied. In a sample of 36 shoots with early shoot spot symptoms observed in Alma, Georgia, in 2015, 72% of shoot spots resulted in complete girdling of the affected shoots. Subsequent dissection of shoot spots showed that tissue damage extended from the epidermis into the pith of the stem (Figure 5E). At this time there is no evidence to suggest that the pathogen overwinters in the affected shoots.
CAUSAL ORGANISM
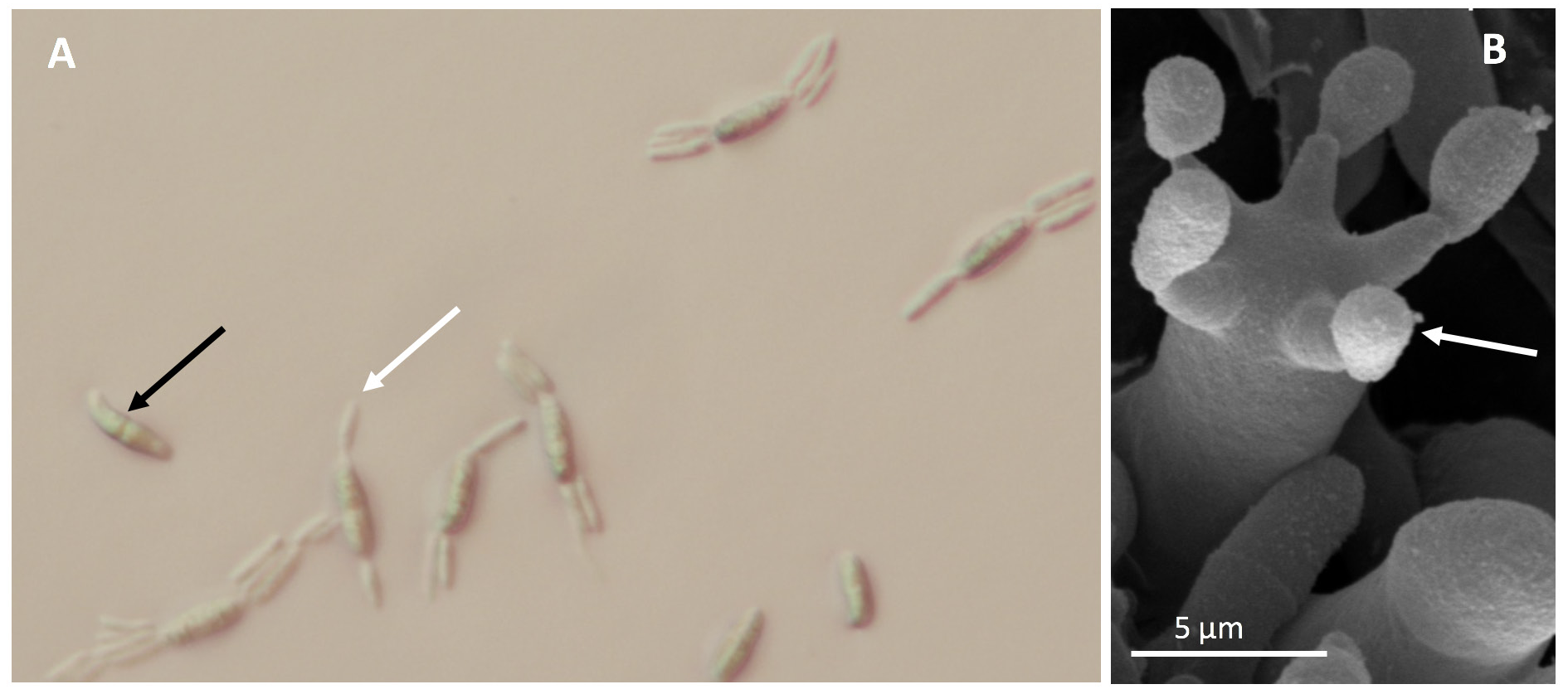
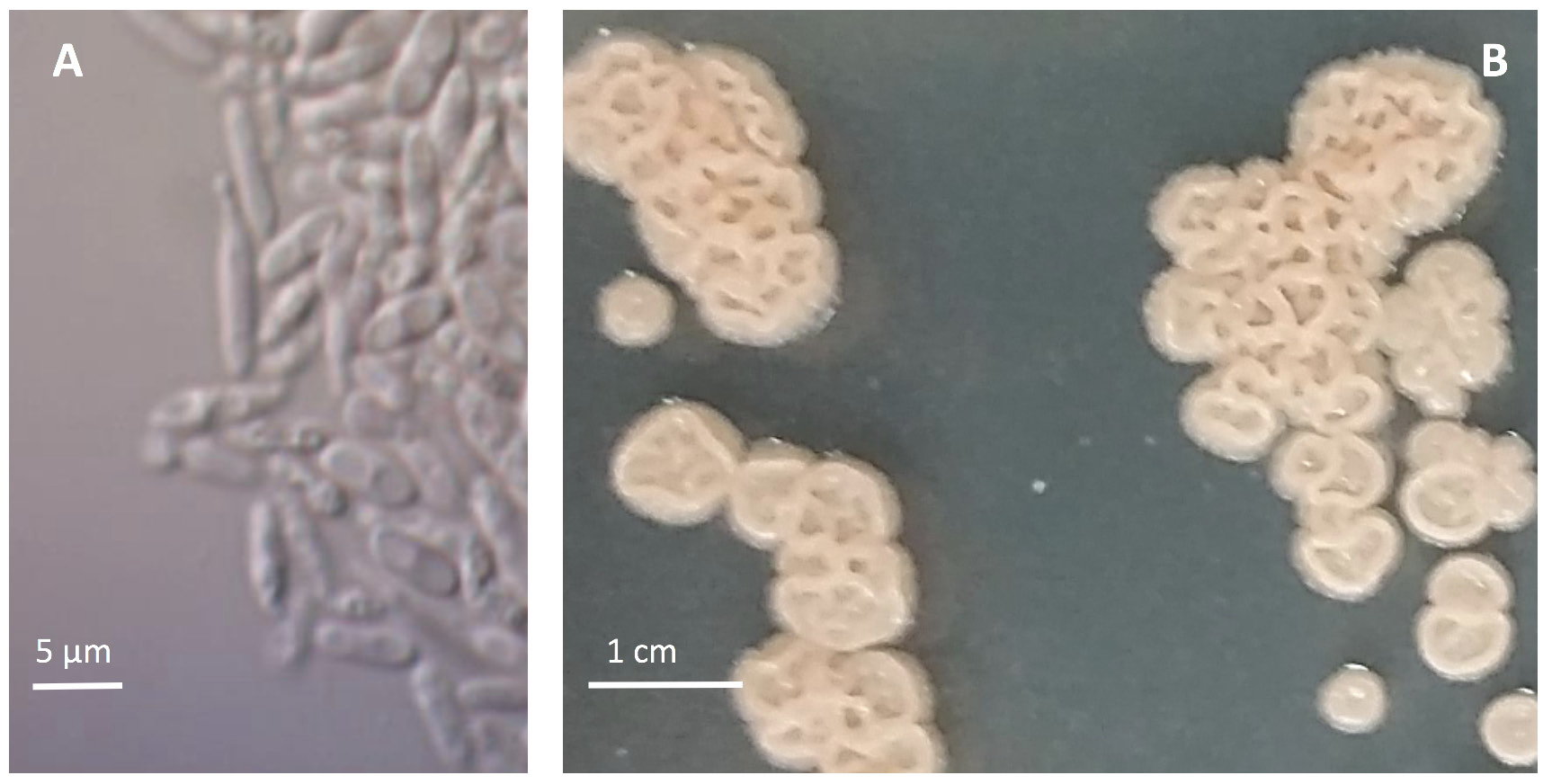
The basidiomycete fungus E. maculosum produces two spore types—sexual basidiospores (Figure 6A) and asexual yeast-like conidia (Figure 7A). The basidiospores are produced on fruiting bodies called basidia (Figure 6B) that can be found on all symptomatic tissues once disease spots reach maturity. County Extension offices can diagnose this disease either directly through in-office examination or via shipment of fresh leaf and fruit samples to Extension diagnostic clinics. Incubating samples has not been effective at inducing sporulation, and therefore, microscopic diagnosis of the disease is most easily conducted by identifying characteristic basidiospores and basidia found on the underside of leaves within actively sporulating disease spots (Figure 6A and B). In the absence of sporulating spots, diagnosis can be conducted via culturing on semiselective media and/or by using species-specific real-time polymerase chain reaction (PCR) primers. Note: E. maculosum has a characteristic raised, wrinkled, and cream-to-pink colony morphology when in culture on potato dextrose agar and the semiselective medium, M5 (Figure 7B).
Photos: M. Brewer, UGA Department of Plant Pathology
DISEASE CYCLE AND CAUSAL CONDITIONS
Exobasidium leaf and fruit spot of blueberry is an early-season, cool-weather disease. In southern Georgia, leaf symptoms typically first appear in late March to early April following leaf emergence and full bloom. By the end of June (postharvest), almost all leaf spots will have turned necrotic, and although the pathogen may still be present on plant surfaces, the disease is no longer active in the field. Multiyear data has indicated that E. maculosum overwinters on the surface of buds and shoots. The overwintering and subsequent source of primary inoculum is most likely the yeast-like conidia (Figure 7A). The window of new infections extends from just after bud break, usually early March until early April, but this window can vary according to plant developmental stage and the occurrence of conditions favorable for disease development. Initial symptoms appear one to two weeks after the infection of recently emerged leaves and young, green fruit. Within one to two weeks after the appearance of symptoms, spots develop to maturity and sporulate to produce clear, curved basidiospores (Figure 6A). In other related Exobasidium spp., it has been suggested that the basidiospore is the infective spore; however, in the case of E. maculosum causing Exobasidium leaf and fruit spot of blueberry, this has not been found to be the case. Season-long monitoring over several years indicated that the vast majority of new infections occurred prior to the appearance of sporulating leaf spots in the field. Furthermore, during periods when sporulation of disease spots was the highest, there were often little or no new infections occurring on leaves or fruit. Artificial infection trials, along with microscopy, must be conducted to confirm this hypothesis, but current evidence suggests that basidiospores primarily serve as the means of long-distance aerial dispersal. Once basidiospores are dispersed, they then likely germinate to produce the yeast-like spores that over summer and overwinter on the surface of blueberry bushes.
Infection of new growth has been associated with extended periods of rain and/or cloudy weather (greater than three days). The association of infection with extended rain events may suggest that the primary inoculum for the disease is splash-dispersed. Susceptibility trials have also indicated that only young leaves, prior to hardening off, and young, green fruit are susceptible to infection. The stage of susceptibility for shoot infection is not known, but it is currently assumed to be specific to young, unlignified (not yet woody) shoots.
CULTURAL CONTROLS
Although no specific cultural controls have been investigated for Exobasidium leaf and fruit spot of blueberry, management strategies for other economically important Exobasidium spp., such as the causal agent of blister blight of tea, Exobasidium vexans, suggest that removing trees on field perimeters and increasing drainage to reduce standing water in problematic locations can be beneficial in reducing disease. Any practice that increases air flow and results in the rapid drying of stems, leaves, and fruit will likely result in less infection; among these, adequate summer pruning and winter basal pruning are critical. Using drip or emitter irrigation will not wet foliage or fruit, whereas overhead irrigation may prove to be problematic because of the possibility of splash dispersal of the pathogen and the system’s potential to create ideal conditions for new infections. Although a comprehensive and reliable list of susceptible versus resistant varieties has not been developed, there appear to be significant differences in the E. maculosum susceptibility of specific varieties. Where ‘Premier’ and ‘Tifblue’ are known to be particularly susceptible rabbiteye varieties, the southern highbush varieties ‘Star’ and ‘Legacy’ have also been reported to experience significant disease on occasion. Although we do not have proof to-date, there is no reason to assume that the field-to-field movement of E. maculosum spores on contaminated equipment would not occur, so it’s encouraged to thoroughly clean equipment (harvesters, hedgers, tractors, etc.) before moving to another field site.
CHEMICAL CONTROLS
Current recommendations suggest that producers apply calcium polysulfide (lime sulfur and similar products) during the late-dormant period of plant development (approximately two weeks prior to bud break; blueberry phenology stage 2). Several studies in Georgia have indicated that even a single application of lime sulfur during this period of development has the potential to almost completely control in-season disease on both leaves and fruit. The effectiveness of lime sulfur at controlling Exobasidium leaf and fruit spot is likely due to the fungicide’s ability to markedly decrease infective spores harbored on the surface of dormant plants. Other fungicides, such as captan or fenbuconazole, have been shown to be moderately effective at providing in- season disease protection when applied at leaf bud break and during bloom. This in-season application window overlaps with that of the chemical control of mummy berry (caused by Monilinia vaccinii-corymbosi), so the application of captan and fenbuconazole between leaf bud break and the end of bloom can serve a dual purpose. It is important to note that there has been a report of E. maculosum resistance to the fungicide Pristine (boscalid + pyraclostrobin) in at least one commercial field in southern Georgia. Given that resistance does occur, the application of Pristine alone for mummy berry management is not recommended for varieties susceptible to Exobasidium leaf and fruit spot. Instead, apply Pristine in a tank mix with captan (check labels carefully, as Pristine labels may preclude tank-mixing in some states). In addition to mummy berry sprays, two to three additional sprays of captan during early cover sprays may be advisable for particularly susceptible varieties. Applying dormant oil for scale insects may actually increase the incidence of Exobasidium leaf and fruit spot of blueberry. In cases where susceptible varieties are sprayed with oil, it is critical that a good Exobasidium fungicide program is in place. Always follow the instructions on the label, and contact your local University of Georgia Cooperative Extension county office for management updates and specific chemical recommendations.
References:
Brannen, P., Cline, B., Scherm, H., & Brewer, M. (2013). Exobasidium leaf and fruit spot development in southeastern environments and initial management strategies with fungicides. Proc. 47th Annual Open House & Trade Show. Clinton, NC: North Carolina Blueberry Council, 5-14.
Brannen, P., Scherm, H., Deom, C., & Noe, J. (2014). New and emerging blueberry diseases in the southeastern United States. Proc. 48th Annual Open House & Trade Show. Clinton, NC: North Carolina Blueberry Council, 25-33.
Brannen, P., Scherm, H., & Allen, R. (2017). Management of Exobasidium leaf and fruit spot disease of blueberry. Acta Hortic., 1180, 215-220. https://doi.org/10.17660/ActaHortic.2017.1180.28
Brewer, M., Turner, A., Brannen, P., Cline, W., & Richardson, E. (2014). Exobasidium maculosum, a new species causing leaf and fruit spots on blueberry in the southeastern USA and its relationship with other Exobasidium spp. parasitic to blueberry and cranberry. Mycologia, 106, 415-423. https://doi.org/10.3852/13-202
Cline, W. (1998). An Exobasidium disease of fruit and leaves of highbush blueberry. Plant Disease, 82, 1064. https://doi.org/10.1094/PDIS.1998.82.9.1064B
Ingram, R., Scherm, H., & Allen, R. (2017). Symptomology and epidemiology of Exobasidium leaf and fruit spot of blueberry. Acta Hortic., 1180, 205-214. https://doi.org/10.17660/ActaHortic.2017.1180.27
Kerr, A., & Rodrigo, W. (1967). Epidemiology of tea blister blight (Exobasidium vexans): IV. Disease forecasting. Trans. Br. Mycol. Soc., 50, 609-614. https://doi.org/10.1016/S0007-1536(67)80092-5
Status and Revision History
Published on Sep 07, 2018
Published with Full Review on Sep 08, 2022


























































