Introduction
Take-all root rot (TARR) was first described as “bermudagrass decline” and later renamed “take-all root rot.” It is also referred to as “root decline of warm-season grasses.” TARR has emerged as a destructive disease in the central, south, and coastal areas of Georgia. TARR affects all warm-season turfgrasses in Georgia, but it is most common and severe in St. Augustinegrass (Stenotaphrum secundatum). The disease is stress-derived, where high soil pH, soil compaction, improper fertility, herbicide injury, and/or moisture imbalances can predispose turf to infections by the fungal causal agent. In Georgia, TARR is most prevalent during moist and warm conditions in the early spring and summer.
The Pathogen
TARR is caused by the fungus Gaeumannomyces graminis var. graminis (Ggg). The fungus forms dark-brown to black runner hyphae on roots, stolons, and rhizomes of hosts (Figure 1). Penetration into the plant and infection are initiated from surface-anchoring structures called hyphopodia (Figure 2). These diagnostic fungal structures can be easily seen under a dissecting microscope on roots, stolons, and rhizomes (Figure 3).
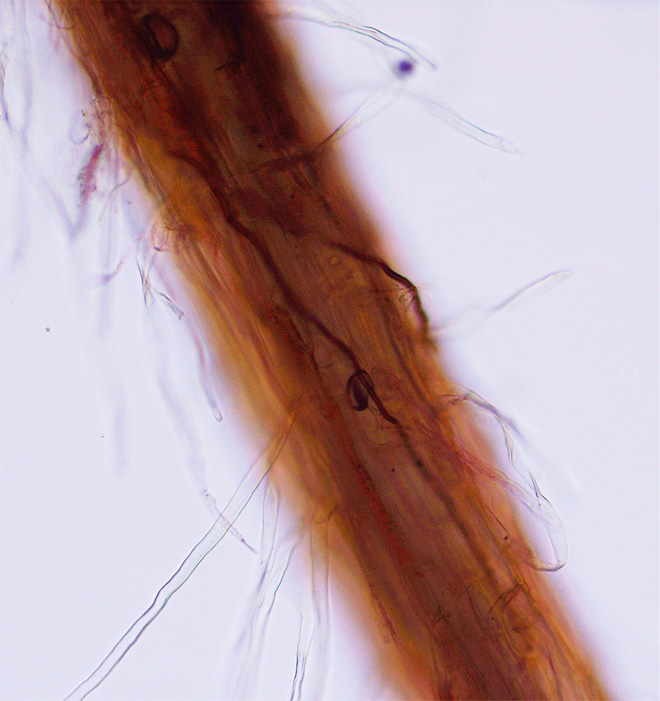
Figure 1. Gaeumannomyces graminis var. graminis hyphae infecting roots of St. Augustinegrass (Photo A. Martinez).
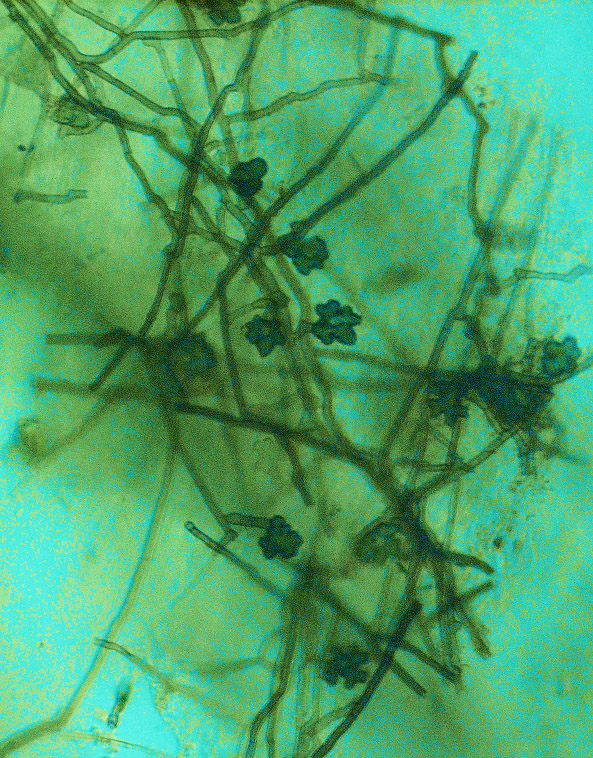
Figure 2. Characteristic Gaeumannomyces graminis var. graminis lobed hyphopodia (Photo A. Martinez).
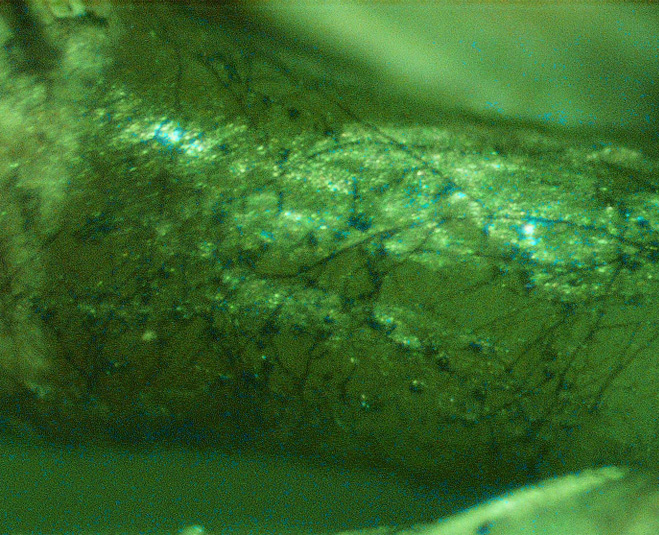
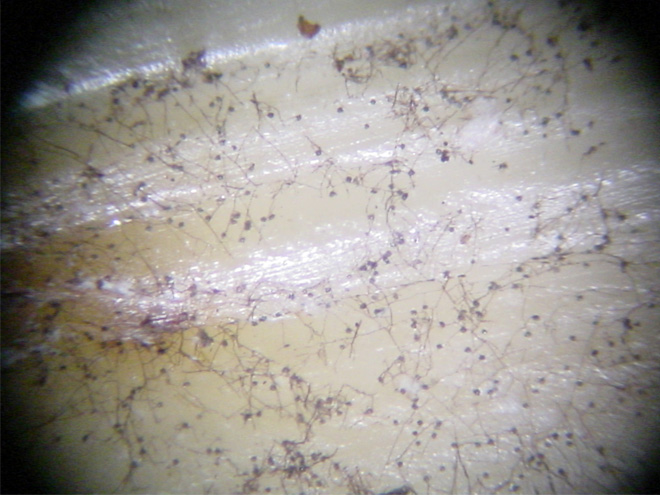
Figure 3. Hyphopodia and mycelium of Gaeumannomyces graminis var. graminis growing on stolons of St. Augustinegrass (Photos A. Martinez and J. Price).
The fungus colonizes roots, stolons, and rhizomes in the form of mycelium, and spreads plant-to-plant by growing along the surface of these tissues. Ggg can also be spread greater distances by movement of these infected plant parts when coring, vertical mowing, or transporting turfgrass. The pathogen does not appear to be spread by mowing or other maintenance implements unless roots and/or stolons are transported.
Symptoms and Signs
The disease first appears as irregularly shaped, yellow, chlorotic areas measuring up to 2 feet in diameter (Figures 4A-B). Lower leaves are the first to become chlorotic, but as the disease progresses, the lower leaves die and upper leaves appear yellow and chlorotic. By the time symptoms are evident on the foliage, the roots, stolons, and rhizomes will be discolored, dark brown and rotted (Figure 5). If severe, the disease appears as dead patches, which quickly merge to form large affected areas (Figure 4B).

Figure 4A. Symptoms of take-all root rot on St. Augustinegrass (Photo A. Martinez).

Figure 4B. Symptoms of take-all root rot on St. Augustinegrass (Photo J. Price).

Figure 5. Take-all root rot symptoms, including yellowing leaf blades and dark brown rotted roots (Photo J. Price).
Conditions that Favor TARR
Warm to hot temperatures (77 to 90 degrees Fahrenheit) and poorly drained soils favor the growth and spread of Ggg. TARR infection is associated with soil measured to a depth of 4 inches with a pH above 6.5. As soil pH increases, availability of many essential trace elements (like manganese, iron, copper, and zinc) decreases and nutrient deficiencies may occur. Light textured soils with low organic matter content and unbalanced fertility also favor the disease. Other organisms, pathogens, and/or environmental stresses such as soil compaction, poor drainage, shade, and herbicide injury may contribute to the enhancement of TARR symptoms.
Control
Genetic
Currently, no resistant cultivars of any of the warm-season grasses are available. One of the most effective means for management of TARR is establishing a turfgrass species well adapted to your geographical area, situation, or landscape. In Georgia, TARR is particularly severe on St. Augustinegrass, so replacing this grass species with other warm-season grasses is one way to avoid the disease.
For example, TARR-affected St. Augustinegrass areas in Brunswick, Georgia, have been replaced with zoysiagrass. Your local county agent can provide you the most updated information concerning turfgrass species and cultivar selection for your area and particular situation. The National Turfgrass Evaluation Program (www.ntep.org) is an excellent resource for information on turfgrass species and turfgrass cultivars.
Cultural
The disease tends to be more severe in soils with high pH. If soils have a pH higher than 6.5, use sphagnum peat moss (pH 4.4) to lower the soil pH by topdressing with 1 bale (at 3.8 cubic feet) for every 1000 square feet. Turfgrass grown in soils with low levels of available manganese (Mn) have shown an increased susceptibility to Ggg. Manganese is less available to turfgrass as pH increases above 6.6. A granular fertilizer containing MnSO4 should be applied if recommended by a recent soil or leaf tissue test.
Any stress placed on turfgrass can promote or intensify TARR. To avoid stress:
- Administer proper plant nutrition and thatch management.
- Have a soil test done and correct fertility according to the test.
- Avoid nitrogen applications in the fall.
- Core-aerate to reduce thatch and relieve soil compaction.
- Do not apply lime to turfgrass areas diagnosed with TARR;
For complete and up-to-date information on fertility for warm-season grasses, consult your UGA Extension county agent, visit www.georgiaturf.com, or see the yearly updated UGA turfgrass pest control recommendations for professionals.
Irrigation: If rainfall is inadequate, irrigate by watering infrequently but deeply. Excessive moisture in the soil and on leaves promotes TARR infection. Irrigating in the late afternoon or evening should be avoided, as this prolongs periods of leaf wetness.
Mowing: Mow the turf at the correct height for the designated turfgrass species and remove only one-third of the leaf blade per mowing. Thatch layers should be removed if they are greater than 1 inch in depth. Vertical mowing is best to remove thatch. Topdressing with sand or soil is less effective than vertical mowing but will help with thatch removal. Controlling thatch can improve drainage, reduce drought and nutrient stress, and remove sources of TARR inoculum.
Herbicides: Avoid activities that stress turf such as herbicide applications. Even when herbicides are applied correctly, St. Augustinegrass is especially sensitive to some herbicides. If possible, manage weeds using cultural management techniques and minimal amounts of herbicides. Herbicides should always be applied according to the label instructions.
Chemical
TARR is difficult to manage once the disease is established.
Homeowners: Due to the stress-derived nature of TARR, emphasis should be placed on cultural rather than chemical controls. For a complete list of homeowner fungicides, refer to the homeowner edition of the Georgia Pest Management Handbook at https://extension.uga.edu/publications/detail.cfm?number=SB48.
Professionals: If TARR is confirmed to be present, preventive fungicide applications may be of benefit after cultural controls have been implemented. Fungicides are more efficacious when applied preventatively rather than after symptoms are evident. Fungicides that contain azoxystrobin, pyraclostrobin, tebuconazole, propiconazole, triadimefon, thiophanate methyl, pyraclostrobin + fluoxapyroxad, pyraclostrobin + boscalid, pyraclostrobin + triticonazole may help control TARR. These materials should be applied with at least 3 gallons of water per 1000 square feet or watered in after application to ensure that the active ingredients reach the root zone. For a complete and updated list of available fungicides, refer to the commercial edition of the Georgia Pest Management Handbook at http://extension.uga.edu/publications/detail.cfm?number=SB28 or the UGA turfgrass pest control recommendations for professionals.
Summary
TARR has emerged as a destructive disease in the central, southern, and coastal areas of Georgia. The disease is stress-derived, where high soil pH, soil compaction, improper fertility, herbicide injury and/ or moisture imbalances can predispose turf to infections by the fungus. The disease first appears as irregularly shaped, yellow, chlorotic areas. Lower leaves are the first to become chlorotic, and by the time symptoms are evident on the foliage, the roots, stolons, and rhizomes will have already been infected and rotting. Conditions that favor growth and spread of Ggg include warm to hot temperatures (77 to 90 degrees Fahrenheit) and poorly drained soils. Soil pH above 6.5 measured to a depth of 4 inches is associated with TARR infection.
Management strategies:
- TARR is particularly severe on St. Augustinegrass. Establishment of a turfgrass species best adapted to your geographical area, situation, or landscape is one the most effective means for managing TARR.
- Administer a soil test and ensure proper fertility according to the provided recommendations. Proper plant nutrition and thatch management will manage the disease by reducing plant stress.
- Core-aerate to reduce thatch and relieve soil compaction.
- Thatch layers should be removed if they are greater than 1 inch in depth.
- Because the disease tends to be more severe in soils with high pH, do not apply lime to turfgrass areas diagnosed with TARR. If soils have a pH higher than 6.5, use sphagnum (pH 4.4) to lower the soil pH by topdressing with 1 bale (at 3.8 cubic feet) for every 1000 square feet.
- If irrigation is used, water infrequently but deeply. Excessive soil moisture will promote the disease.
- Mow the turf at the correct height for the designated turfgrass species and remove no more than one-third of the leaf blade per mowing.
- Few effective fungicides are available for homeowners to manage TARR, so the emphasis should be placed on cultural control.
- A variety of fungicides are available for professional turfgrass managers for TARR. Fungicides should be used in combination with cultural controls.
References
Colbaugh, P. F., Williams, E. A., McAfee, J. A., & Heitholt, J. J. (2005). Use of sphagnum peat moss topdressing to control take-all root rot of St. Augustinegrass (Stenotaphrum secundatum). International Turfgrass Society Research Journal,10, 170–174.
Martinez-Espinoza, A., Gardner, D., & Price, J. (2005). Management of Gaeumannomyces graminis (take-all root rot) on St. Augustinegrass in coastal Georgia using fungicides and soil amendments. Phytopathology, 95(6), S66.
National Turfgrass Evaluation Program. https://www.ntep.org
Sial, A., Taylor, M., & Cabrera, E. (Eds.). (2022). Georgia Pest Management Handbook—Home and Garden Edition (Special Bulletin 48). University of Georgia Cooperative Extension. https://extension.uga.edu/publications/detail.cfm?number=SB48
Smiley, R. W., Dernoeden, P. H., & Clarke, B. B. (2005). Compendium of Turfgrass Diseases. American Phytopathological Society. https://doi.org/10.1094/9780890546154
Taylor, M. (Ed.). (2022). Georgia Pest Management Handbook—Commercial Edition (Special Bulletin 28). University of Georgia Cooperative Extension. https://extension.uga.edu/publications/detail.cfm?number=SB28
University of Georgia. Georgia Turf. http://www.georgiaturf.com
University of Georgia. Georgia Turf: Pest Control Recommendations. https://turf.caes.uga.edu/publications/pest-control-recomendations.html
Waltz, C., Martinez-Espinoza, A., Pearce, M., & Martin, B. (2003, April). Fungus Family Provides Fodder for Several Diseases. Turfgrass Trends, 52–56. https://archive.lib.msu.edu/tic/golfd/ article/2003apr52.pdf
Status and Revision History
Published on Oct 18, 2016
Published with Full Review on Jun 24, 2022


























































