Proper fertilization is one of the most cost-effective ways to cultivate an attractive lawn. A regular fertilization program is important to maintain healthy, attractive turf. It should include:
- Applying the correct type, or analysis, of fertilizer;
- Using the correct amount of fertilizer; and
- Fertilizing at the proper time.
To be most effective, fertilization must be combined with proper management practices such as mowing, irrigation, and pest management. For information on proper management and fertilizer timing, visit turf.caes.uga.edu and follow the “Cultural Practices” link to turfgrass management calendars for each turfgrass species grown in Georgia. For information on soil texture, organic matter, aeration, and soil pH, see Circular 1058-1, Soil Texture, Organic Matter, Aeration, and pH: extension.uga.edu/publications/detail.html?number=C1058-1.
Fertilizer Terminology
Turfgrasses require a number of nutrients for growth. Three of these — carbon, hydrogen, and oxygen — are rarely lacking because grasses get them from carbon dioxide in the atmosphere and from water in the soil. The remaining 18 essential elements are also obtained from the soil, but they may not be available in sufficient quantities.
The major elements, nitrogen (N), phosphorus (P), and potassium (K), are those needed in the highest concentrations, and are commonly supplemented with fertilizer. The three numbers on the front of fertilizer bags are often called the “N-P-K numbers.”
Secondary nutrients calcium (Ca), magnesium (Mg), and sulfur (S) are required in lesser concentrations than N, P, and K.
Micronutrients are required in very small amounts, and include chlorine (Cl), iron (Fe), manganese (Mn), boron (B), zinc (Zn), copper (Cu), molybdenum (Mo), sodium (Na), cobalt (Co), silicon (Si), selenium (Se), and nickel (Ni).
A soil test is the best way to find your soil’s nutrient status. Nitrogen is not reported on a typical soil test result, because its presence in the soil is too transient to accurately analyze. Nutrients that are most commonly reported include P, K, Ca, Mg, Mn, and Zn. To have your soil tested, contact your local UGA Extension office, or order a sampling kit at aesl.ces.uga.edu/soil.html.
A fertilizer analysis, or grade, refers to the percentages of nitrogen (as N), phosphorus (as P2O5), and potassium (as K2O) in the fertilizer. A 16-4-8 grade fertilizer contains 16 percent N, 4 percent P2O5, and 8 percent K2O. There are many fertilizer analyses to choose from, but the percentages and calculations are the same.
A complete fertilizer has all three of the major elements present. An example of a complete fertilizer is the 16-4-8 mentioned above because it contains percentages of nitrogen, phosphorus, and potassium.
An incomplete fertilizer contains only one or two of the major elements. An example of an incomplete fertilizer is 15-0-15, which contains only nitrogen and potassium.
A balanced fertilizer contains equal amounts of all three major elements. For example, a 10-10-10 analysis is a balanced and complete fertilizer. Research in soil fertility shows that most lawns in Georgia are lower in potassium than phosphorus, indicating a need for fertilizers containing higher amounts of potassium than phosphorus. Where phosphorus generally does not leach through the soil, potassium may. Fortunately, potassium does not contribute to water or environmental quality issues.
As a result of differing nutrient levels in soil, many turfgrass fertilizers have an unbalanced analysis. For example, 16-4-8 and 15-0-15 are unbalanced fertilizers.
A fertilizer ratio is the ratio of the percentages of N, P2O5, and K2O in the fertilizer. An example of a 1-1-1 fertilizer ratio is 10-10-10. An example of a fertilizer with a 4-1-2 ratio is 16-4-8. To calculate the ratio, take the smallest number in the grade and divide it into each number of the grade. For example, the smallest number in a 16-4-8 grade is 4. It can be divided into 16 four times and into 8 two times, making a 4-1-2 ratio. Therefore, 164-8 = 4-1-2.
The Fertilizer Label
Georgia law requires fertilizer producers to display the guaranteed analysis on the fertilizer container whether they are inorganic or organic fertilizers (Figure 1). The grade of the fertilizer in Figure 1 is 16-4-8. The first number (16) represents the percentage of nitrogen (N); the second number (4) represents the percentage of phosphorus (P2O5); and the third number (8) represents the percentage of potassium (K2O). Many fertilizer labels will list the source of phosphorus as “phosphate” and potassium as “potash.” A 50-pound bag of 16-4-8 fertilizer contains 8 pounds of nitrogen (50 x 0.16 = 8), 2 pounds of P2O5 (50 x 0.04 = 2), and 4 pounds of K2O (50 x 0.08 = 4), for a total of 14 pounds of nutrients. The other 36 pounds of material in the bag is called filler or carrier.
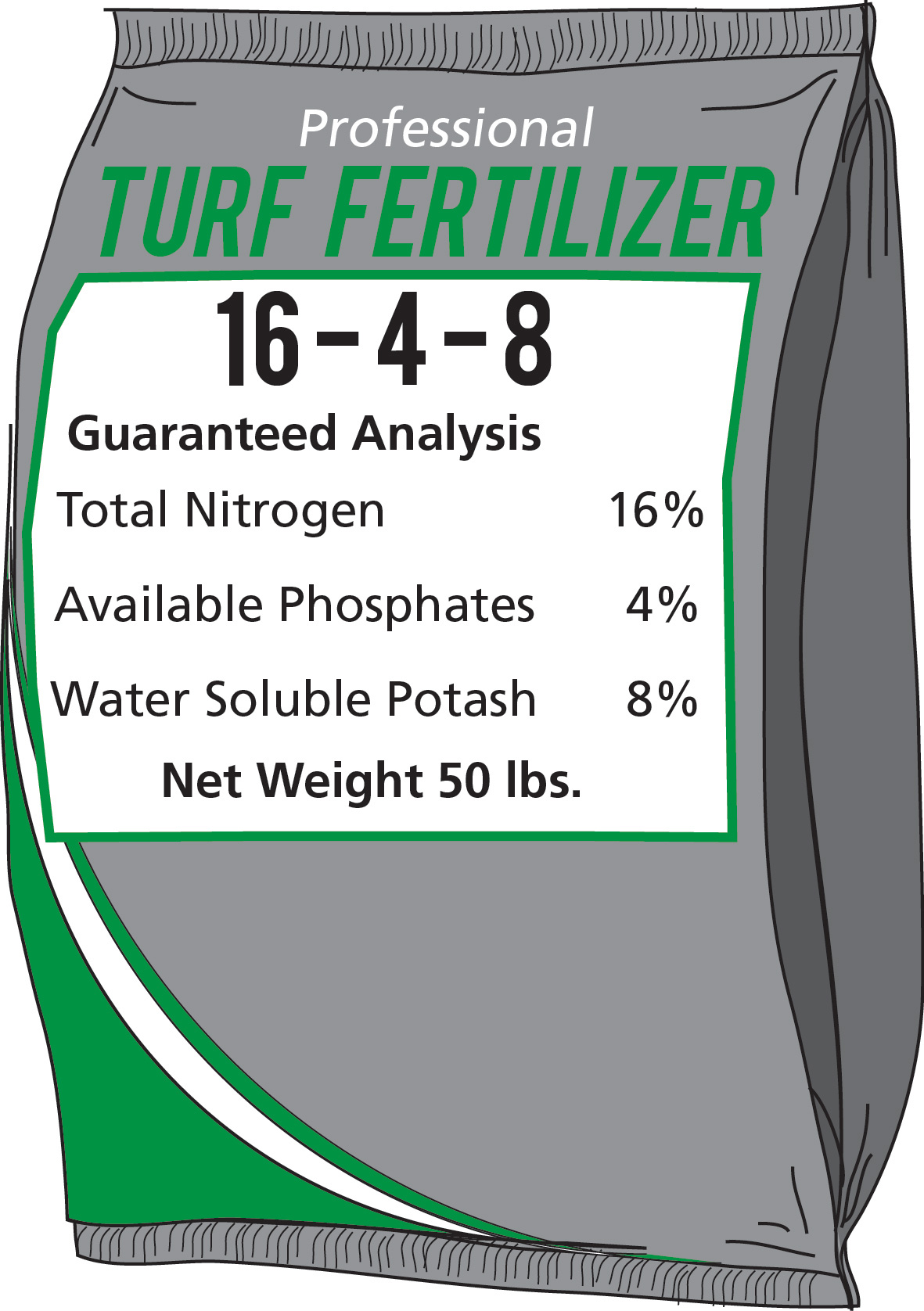
Figure 1. Basic Fertilizer Label
Nitrogen Sources
Nitrogen materials can be divided into two groups. One is called “quickly available” or “water soluble,” and the second is referred to as “slowly available,” “water insoluble,” or “controlled-release.” Provided that the soil is adequately moist, plants can immediately absorb quickly available nitrogen. Quickly available nitrogen typically includes materials like ammonium, nitrate, and urea. In addition, quickly available materials generally:
- Are less expensive;
- Can cause rapid growth;
- Have short soil residual;
- Have the potential to leach through the soil, particularly nitrate; and
- Have high burn, or injury, potential to the plant.
Slowly available materials release nitrogen more gradually and over a longer period of time. The rate of nitrogen release can be dependent on factors such as microbial activity, soil moisture content, and temperature. Examples of slowly available materials include sewage sludge, pelletized poultry litter, feather meal, methylene urea, isobutylidine diurea (IBDU), sulfur-coated urea (SCU), polymer-coated urea (PCU), and ureaformaldehyde (UF). Do not confuse these with urea, which is a fast release material. Slowly available materials generally:
- Are more expensive;
- Require fewer applications;
- Reduce losses to leaching; and
- Have low burn potential.
Slowly available nitrogen is usually identified on the label as “water insoluble nitrogen” (WIN), UF, IBDU, SCU, or poly-coat (PCU). The 16 percent nitrogen in Figure 2 represents the total percentage of nitrogen in the bag. To calculate the percentage of the total nitrogen that is water insoluble, divide the percentage of WIN by the total percentage of nitrogen, and multiply by 100. In the example of the label in Figure 2, we know to divide 8 percent by 16 percent and multiply by 100, where we find that 50 percent of the total nitrogen is water insoluble or slowly available. A slow release lawn fertilizer should contain at least 30 percent of the nitrogen in a slow release form. If 50 percent or more of the nitrogen is in the slow release form, twice the recommended amount of nitrogen can be applied half as often.
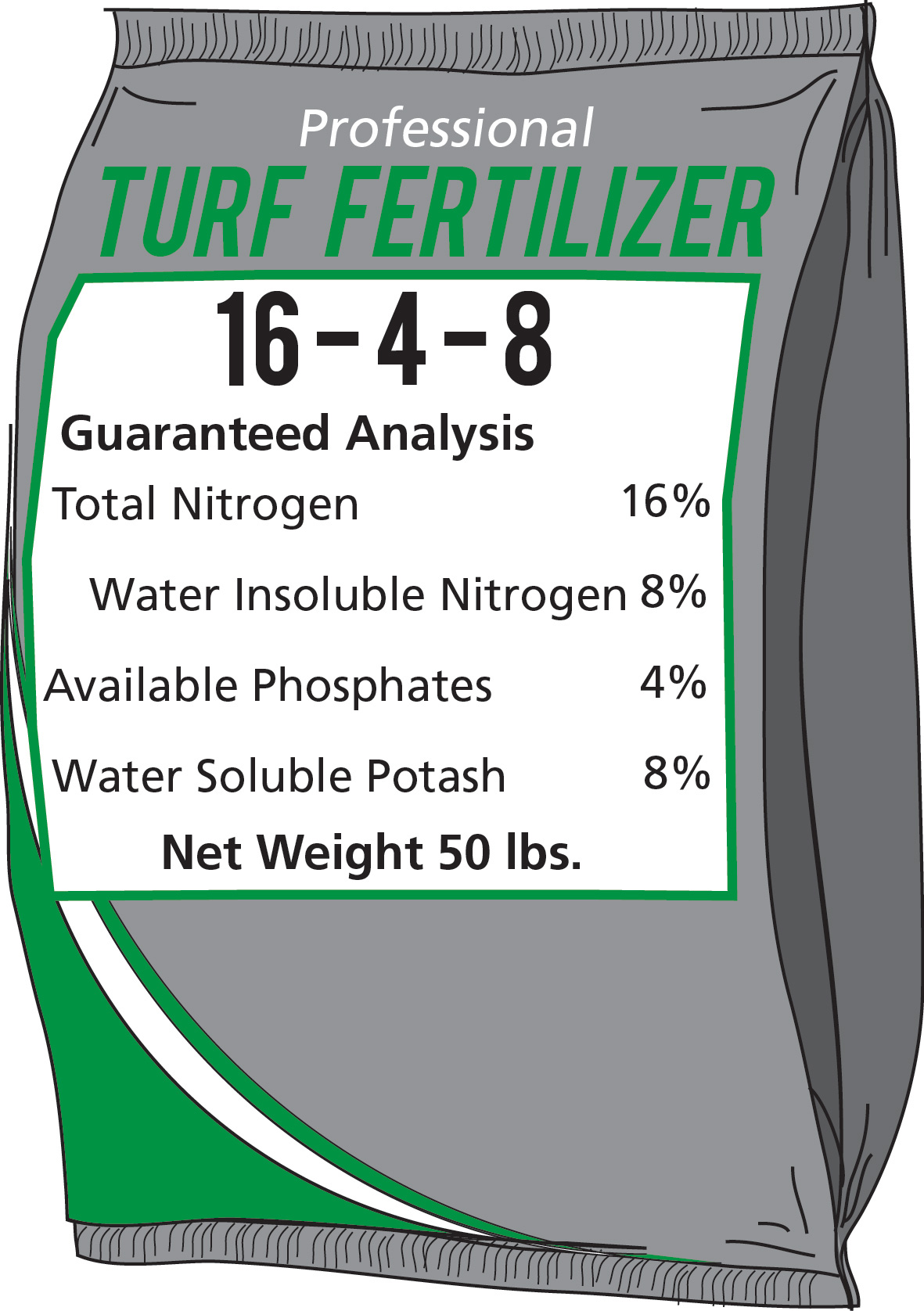
Figure 2. Fertilizer Label with Nitrogen Breakdown
Secondary Nutrients and Micronutrients
The Georgia fertilizer law does not require fertilizer companies to guarantee the secondary or micronutrient content unless it is claimed in their advertisements. In order to determine whether these elements are contained in lawn fertilizer, check the analysis tag. If these elements are in the fertilizer, the tag will generally show the percentage of each. See Figure 3.
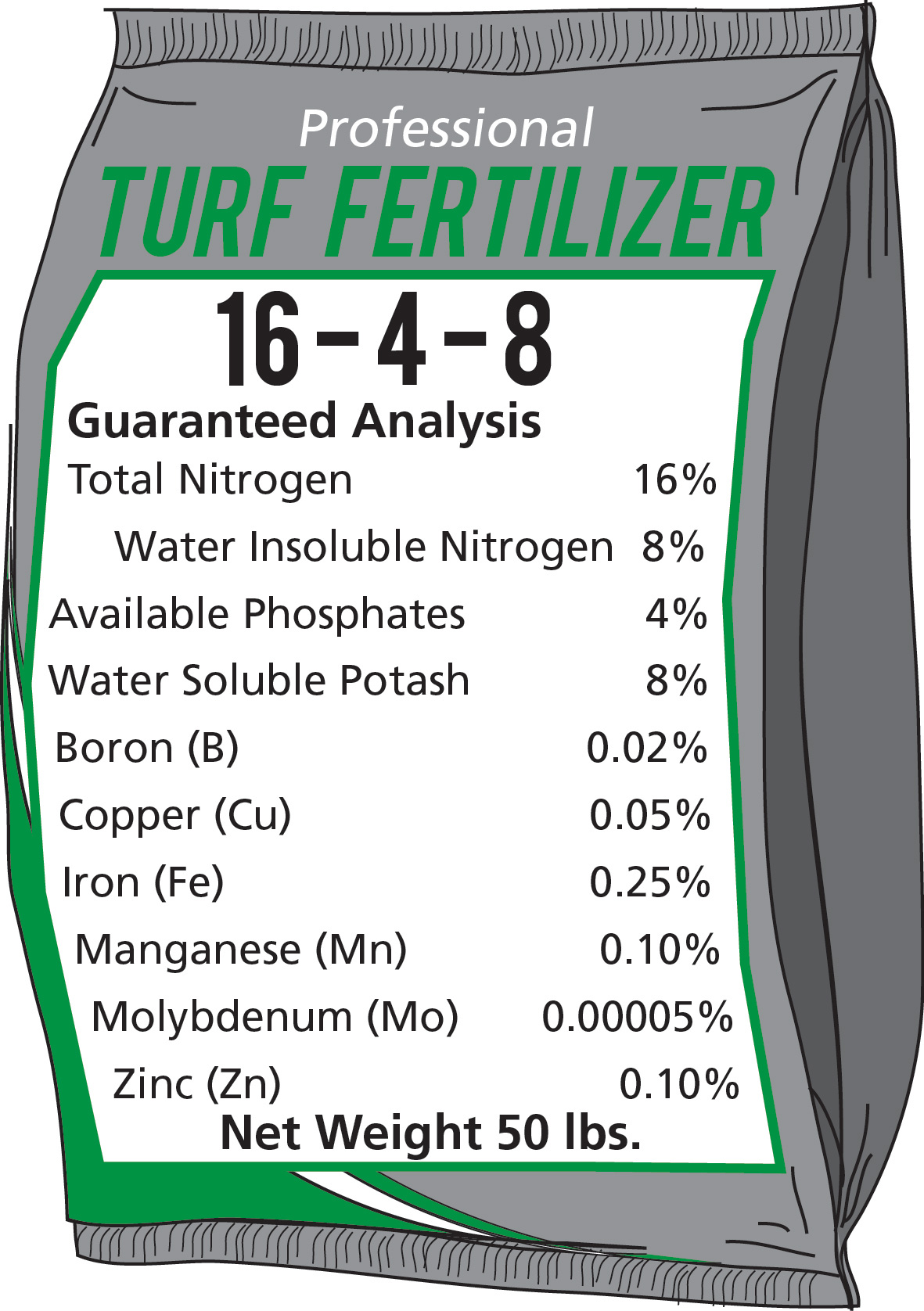
Figure 3. Detailed Fertilizer Label
In the spring, it is not uncommon for some turfgrass species, particularly centipedegrass, to turn yellow. Several factors may cause yellowing, including a lack of iron. In turfgrass, iron (Fe) is probably the most commonly applied micronutrient because it can cause a rapid “greening” of the turfgrass without stimulating excessive growth. Although iron is not an actual component of chlorophyll (the pigment that gives plant leaves their green color), it is essential for plants to make chlorophyll, which is why iron levels affect turfgrass color.
The most common forms of iron fertilizer for turfgrasses are inorganic iron salts and organic iron chelates (chelated iron). Chelate comes from the Greek word “clawlike,” and means that an organic compound, called a chelating agent, is bonded to a nutrient (like Fe) to form a soluble and stable product. The chelating agent keeps the nutrient in solution and releases it either through the turfgrass foliage or at the root surface. Since turfgrasses can absorb iron from these products through the leaf, they are typically applied as foliar sprays. Chelated forms of iron also have the advantage of lasting longer in the soil. This is because the chelating agent protects the iron from being converted into an unavailable form of the nutrient.
Chelated iron sources are usually more efficient at supplying plants with iron than inorganic iron salts (like iron sulfate, FeSO4). Iron sulfate is typically less expensive than iron chelate, and as a result, FeSO4 is more commonly used and applied more frequently. An inorganic iron salt can also be water soluble, and is generally applied as a foliar spray. If not absorbed through the leaf and allowed to reach the soil surface, inorganic salts are converted to insoluble forms of iron, which become unavailable to the turfgrass plant.
Granular fertilizers sometimes contain small amounts of iron, although they are not necessarily in the form readily available to plants. Hence, soil applications of iron generally require high rates to obtain a modest color response. Thus, foliar applications of iron are more effective and more frequently used in eliminating iron deficiency and enhancing green color.
The effect from a supplemental iron application can be noticeable soon after application (within 1 to 2 days) but is temporary (about 2 to 4 weeks). Therefore, repeat applications are necessary for summer-long color. To correct an iron deficiency or “green the turf,” apply ferrous sulphate, ferrous ammonium sulphate, or a chelated source according to label directions. Usually 2 to 4 ounces of product per 1,000 square feet, generally equivalent to 2 pounds of iron per acre, will correct deficiency problems and green-up the grass. Spray applications should be applied at 3 to 5 gallons per 1,000 square feet, and can be easily made with a hose-end sprayer. Excessive amounts of iron can cause a noticeable discoloration — that is, cause grass to turn black — so it’s important to avoid spray overlaps. Iron can also stain sidewalks, driveways, paint, walls, and other surfaces.
Non-traditional, or “Organic,” Fertilizers
Interest in using organic fertilizers on lawns has increased over the past decade. Both organic and inorganic fertilizers have advantages and disadvantages, but the type of fertilizer makes no difference to the grass plant. In fact, plant roots absorb the majority of their nutrients from the soil solution as inorganic ions, which are charged atoms or molecules like NO-3, NH+4, H2PO4-, HPO4-2, K+, etc. Most inorganic fertilizers dissolve in water and are readily available to plants. Organic fertilizers are more complex chemical substances that take time — several weeks to months — to be broken down into forms usable by plants. They are slow release type fertilizers, compared to the quick release characteristics of most inorganic fertilizers. Because of the slow release characteristics of organic fertilizers, turfgrass growth may be gradual over a period of one to two months.
Most organic fertilizers contain between 3 and 10 percent nitrogen. Because more fertilizer material must be applied to achieve a recommended nutrient rate, the cost of organic fertilizers on a nutrient-per-pound basis is usually higher than quick-release inorganic fertilizers.
Organic nitrogen is not used directly by plants; it must first be converted into a usable form. Nitrogen is released from organic fertilizers when naturally occurring soil microbes break down the fertilizer’s organic molecules. Soil temperatures and moisture regulate soil microbial activity. Extended dry or cold periods may delay release of nitrogen from organic fertilizers. While it is important to apply organic fertilizers well before periods of rapid plant growth, when fertilizing with organic fertilizers, expect slower greening in the spring.
Processed poultry litter, feather meal, biosolids, seaweed extracts, corn gluten, and various composts are examples of non-traditional or “organic” lawn fertilizers. Many companies now formulate complete organic fertilizers from a variety of animal byproducts and minerals. These fertilizers have grades such as 8-3-5, 5-6-6, and 12-0-0.
Animal manures and poultry litter may be readily available from local farms. However, care needs to be taken that these materials are composted, as fresh animal manure can carry pathogens, have weed seed, or cause plant burn. Because composted manures can vary widely, these products should be analyzed to determine their nutrient content prior to use on turfgrass as a fertilizer. For additional information on composting, see University of Georgia Cooperative Extension Circular 816, “Composting and Mulching.”
In addition to nutrients, organic fertilizing materials like manure and compost add organic matter to the soil. Organic matter can increase a soil’s water holding capacity, nutrient retention, and aeration. Additionally, the beneficial effects of organic matter on soil structure can have a greater impact on plant growth than the nutrient value of the fertilizer itself.
Again, research nutrient content and application practices before using any of these products. Generally, organic fertilizers are lower in nutrient value than traditional fertilizers, but their nutrients are released more slowly. When used according to recommendation, organic fertilizers can supply the required nutrients for turfgrass growth. However, organic fertilizers misapplied at excessive rates can injure plant roots and lead to environmental problems, just like inorganic fertilizers. As with more traditional fertilizers, organic fertilizers should be part of a larger plan of sound lawn management practices. Remember, the advantages and disadvantages of organic or inorganic fertilizers are more related to the consumer than the grass.
Being familiar with basic fertilizer terminology and understanding how to read a fertilizer label can simplify lawn care. To have a healthy and attractive lawn, apply the correct analysis of fertilizer, use the recommended amount, and fertilize at the proper time.
Status and Revision History
Published on Jul 18, 2016


























































