Introduction
Pierce’s disease (PD) of grape is caused by the bacterium Xylella fastidiosa (Xf ; Wilcox et al., 2015). Xf is a xylem-limited bacterium. “Xylem-limited” means that Xf only inhabits the xylem elements within the host plant. Xylem elements are the tissues that transport water and nutrients from the roots up to the shoots and leaves. Xf limits water and nutrient translocation by persisting and expanding in the xylem elements and causing the plant to produce tyloses, blocking the “pipes” through which those resources can pass. If the Xf bacterium multiplies and expands enough, the result can be complete obstruction of water and nutrient translocation to aboveground vegetative tissues of the grapevine. Vines will consequently die within one to two years of PD diagnosis, which is well before the anticipated 20- to 25-year productive lifespan of a vineyard in the Eastern U.S. There are many biotic and abiotic pests that make wine grape cultivation challenging in the Southeastern U.S. PD can make it impossible to grow many wine grape cultivars in certain sub regions, as it kills them outright. PD is thus a great threat to the sustainability of commercial vineyards located in warm, coastal, and/or subtropical grape growing regions of the Southeastern U.S. as well as regions in Texas and California.
Range and causal conditions
Xf is endemic to the Southeastern U.S. and is a threat to vine sustainability in sub regions (e.g. Piedmont, Coastal Plain, footshills, etc.) with mildwinters. Pierce’s disease can produce significant losses in vineyards from Florida to Virginia, but also in vineyards westward in Alabama, Texas, and Mississippi. PD is becoming increasingly common in susceptible cultivars (V. vinifera; popular hybrid bunch grapes such as Vidal blanc and Chambourcin) planted in northern Georgia and low-altitude North Carolina and Virginia vineyards. The increasing PD prevalence in the Southeastern U.S. is likely a function of milder winter temperatures (Anas et al., 2008; Lieth et al., 2011). PD is generally less prevalent in vineyards located in northern, high altitude regions on the East Coast (Pennsylvania, New York) as well as inland regions that experience frigid winter temperatures, such as the Midwestern U.S. (Indiana, Michigan, Ohio). Pierce’s disease has been identified, however, in Oklahoma and Missouri (Smith, 2019). It has generally been recommended that producers in Georgia not plant PD-susceptible vines at elevations below 1,300 ft above sea level, and rarely has PD been observed at elevations above 2,000 ft. However, PD has recently been observed to cause significant losses in vineyards at and above 1,300 ft; after warm winters, PD has even been confirmed in vineyards planted above 2,000 ft in northern Georgia, as well as in lower altitude vineyards in the North Carolina foothills. This indicates that the 1,300 ft elevation limit is now at a higher elevation.
Symptoms and identification
As with several systemic grapevine diseases and nutrient imbalances, PD symptoms often become evident around veraison, the growth stage characterized by berry softening, sugar accumulation, and coloration (in red-fruited cultivars). Veraison is also the growth stage at which carbon resource allocation is shifted from the canopy vegetation to the ripening fruit; stressors become visually manifested in the canopy thereafter. Veraison typically occurs in mid- to late- July in many Georgia and Southeastern U.S. vineyards, but this will vary by cultivar, vintage, and growing region. Scouting for PD should begin in mid-July in those regions. Symptoms will occur earlier in drought years, but symptoms are generally most evident from late August through September. In grapevines, Xf causes four visual symptoms that together are strongly associated with PD. Leaf necrosis is observed on the margins of leaves (Figure 1A). Marginal leaf necrosis can be a common response to several vineyard issues; its presence alone should therefore not alarm growers. However, growers are encouraged to contact local Extension agents if the following symptoms are observed in tandem with marginal leaf necrosis. “Matchstick” is the term used to describe the symptom that occurs when only the leaf blade abscises and the petiole (leaf stem) remains attached to the shoot (Figure 1B). “Green islands” describes the phenomenon observed when the shoot tissues harden off unevenly, resulting in green, vegetative “islands” within the brown, woody tissue (Figure 1C). Lastly, as xylem flow is increasingly blocked by bacteria, infected plants will also form “raisins” as the fruit clusters dry out from lack of translocated water (Figure 1D).




Management
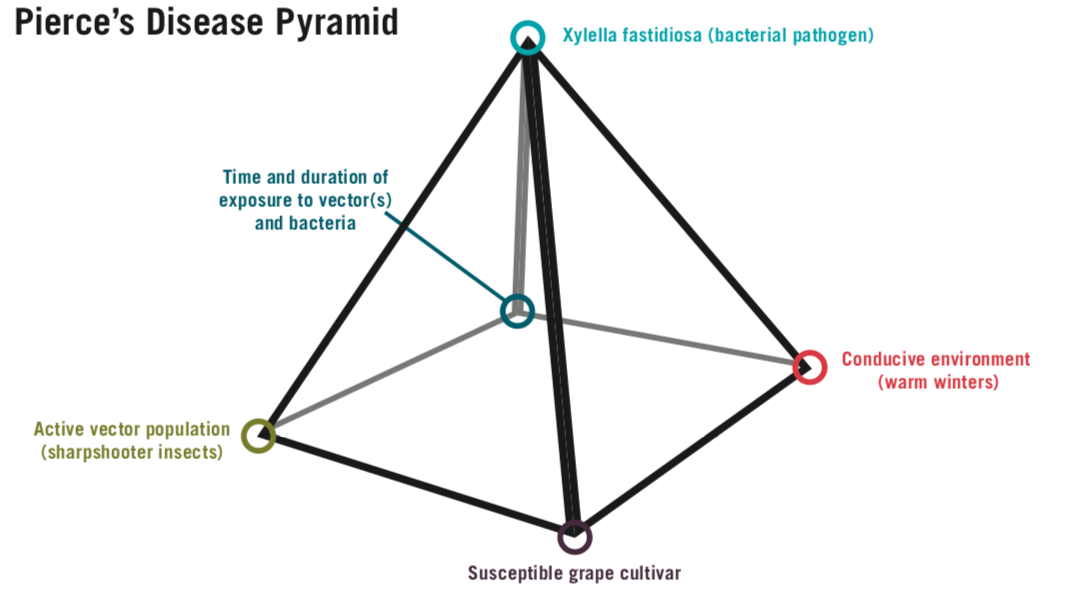
The disease pyramid shows the necessary elements for grapevine PD to occur: a susceptible host, a conducive environment, a vector, an aggressive pathogen, and appropriate timing of vector feeding and bacterial ingress (Figure 2). A subfamily of leafhopper insects called “sharpshooters” are the significant means by which Xf is introduced into grapevines in the Southeastern U.S. Though pruning and root grafting are possible means of infection, these are considered to be of limited importance (Krell et al., 2007). For this reason, without the presence of sharpshooter vectors, grapevine PD is unlikely to occur. The grapevine PD pyramid provides a good basis to develop a PD management plan in vineyards; exclusion or management of any of the five elements shown in the triangle below will result in successful, proactive grapevine PD management.
Annual dormant pruning has been anecdotally shown to aid in PD management in a commercial vineyard in Dahlonega, Georgia; failure to prune can exacerbate premature vine death due to PD. While there is no guarantee that pruning will remove all infected grapevine wood, annual winter pruning of 1-year-old canes can aid in PD management by removing first-year localized Xf infections (Varela et al., 2001). Pruning may particularly aid in managing Xf infections that have occurred relatively later in the growing season, and thus have had a lesser chance of colonizing permanent vine parts before vines enter dormancy, relative to those infections that have occurred earlier in the season (Varela et al., 2001). Indeed, vines infected by Xf earlier in the season (April and May) showed more severe PD symptoms and had less recovery rate relative to vines infected later in the season (June through August)
(Feil et al., 2002). While extremely impractical, cleaning tools with a 10% bleach solution can help reduce mechanical transmission of Xf to uninfected vines, particularly when hedging or trimming canopy shoots during the growing season (Smith, 2019). Once a vine is diagnosed to be PD-positive, the only 100% effective, retroactive management strategy is to rogue and remove the vine from the vineyard. PD symptoms are typically expressed later in the season,and identification may thus coincide with harvest time. If harvest activities preclude the immediate removal of infected vines, it is advised that infected vines be flagged so they can be easily identified for removal after the harvest is complete.
The only way to “manage” a PD-conducive environment is to plant vineyards in northern regions or at elevations where winter temperatures are low enough to prevent the survival of Xf in the plant (Anas et al., 2008; Feil and Purcell, 2001). Population numbers of key insect vectors may also be reduced in colder environments, but this impact of cold winters is likely secondary to reduced bacterial survival. Environments characterized by high and low PD threat can be found within the same state and are generally separated by a vague line of division that moves over time and between vintages. For example, the Texas Hill Country and north Georgia mountains are planted with PD-susceptible hybrid and vinifera cultivars, while the Texas Gulf Coast and Georgia Piedmont are planted with PD-tolerant cultivars.
Planting grapevine cultivars known to be tolerant to Xf is the best way to proactively manage PD in environments that are conducive to sustained Xf exposure. Tolerant cultivars are generally void of symptoms and their productivity is unaffected when planted in PD-conducive environments. Most muscadine cultivars and several hybrid wine grape cultivars (e.g. ‘Blanc du Bois’ in Figure 3A, ‘Villard blanc’ in Figure 3B, ‘Lenoir’ in Figure 3C, ‘Norton’, etc.) tolerate Xf and therefore do not often show PD symptoms. While ‘Villard blanc’ is generally accepted as “PD-tolerant,” growers have reported visual PD symptoms in Villard blanc vineyards in the Southeastern U.S., and the same is true of Norton if grown under heavy PD pressure. Several T.V. Munson cultivars are also PD-tolerant (Kamas et al. 2010), the most popular of those being ‘Lomanto’ (Figure 3D).
‘Crimson Cabernet’, a privately bred, publicly available, red-berried cultivar developed from crosses between ‘Norton’ and ‘Cabernet Sauvignon’, has anecdotal PD-tolerance. However, some vines of 'Crimson Cabernet' planted in the Georgia Piedmont have shown some symptoms and tested positive for Xf within 10 years of planting, indicating that this cultivar may not be suitable in very high-PD risk environments.
High V. vinifera-parentage selections from Professor Andy Walker’s breeding program at University of California–Davis tolerate PD and produce vinifera-like quality wines. Two white and three red selections with 94% (‘Camminare Noir’) or 97% (‘Ambulo Blanc’, ‘Caminante Blanc’, ‘Errante Noir’, and ‘Paseante Noir’) vinifera genetics have been commercially released. Field trials are currently underway in Georgia and elsewhere in the Southeast to evaluate the growth and performance of ‘Crimson Cabernet’ and UC Davis selections.




The PD-causing Xf is vectored and transmitted by xylem-feeding sharpshooters (Cicadellidae: Cicadellinae) and spittlebugs (Cercopidae) which feed on green, vegetative shoots of the grapevine. Many xylem-feeding leafhoppers, such as the broad- headed sharpshooter (Oncometopia orbona), versute sharpshooter (Graphocephala versuta), and the red- banded leafhopper (Graphocephala coccinea), are found in the Southeastern U.S. and can transmit the Xf bacterium (Krell et al., 2007; Myers et al., 2007; Redak et al., 2004). The versute sharpshooter is commonly found in northern Georgia (Figure 4).
The glassy-winged sharpshooter, Homalodisca vitripennis (Germar) (Hemiptera: Cicadellidae), is one of the most important vectors of Xf in the Southeast (Figure 4). This large, 0.5-in.-long sharpshooter is native to the Southeast, and its bulbous head and thorax (middle body segment) are dark brown with yellow dots. Its face, eyes, and legs are yellow-orange with dark markings. The glassy-winged sharpshooter has translucent, smoky brown, and membranous wings that cover its abdomen, which is white with black markings. A publication by Varela et al., (2001) provides a thorough review of Xf insect vectors.
Sharpshooters feed and reproduce on a wide range of plants in a variety of habitats and prefer succulent over lignified growth, so grapevines are ideal sharpshooter habitats because they produce copious amounts of succulent vegetative growth. The glassy-winged sharpshooter is a strong flier relative to other sharpshooters, so it can disperse across the landscape and easily move into the vineyard and behind vineyard edges that are adjacent to forested habitats. With its high propensity for movement and its dark coloration, the glassy-winged sharpshooter is very inconspicuous in the wild. Additionally, it often hides by moving to the other side of the cane when it detects movement or is disturbed. Thus, visually inspecting vines for glassy-winged sharpshooters can be tricky and ineffective, so using yellow sticky traps is a better tactic.
The best time to start trapping for sharpshooters is at bud break. Monitoring glassy-winged sharpshooters at this time will help determine activity and movement of sharpshooters into the vineyard from surrounding habitats. Use large, double-sided yellow sticky traps, no smaller than 4 by 7 in., that contain plenty of sticky material (Varela et al., 2001). Hang traps every 150 ft at canopy height throughout each block (area of contiguous rows of vines). Hang traps at the interface of the vineyard and all adjacent habitats, particularly forested and riparian borders. Check traps weekly and remove the insects from traps after counting them. Replace the yellow sticky traps at least once a month or when they are otherwise rendered inefficient at trapping and permitting efficient insect counting and identification. Continue monitoring for the glassy-winged sharpshooter throughout the season until daytime high temperatures remain below 65 °F (Varela, 2001).
The management of sharpshooters has been shown to suppress PD epidemics. One way to manage sharpshooters is to manage the habitat in which they feed and breed. However, such attempts may prove futile because sharpshooters have a very wide range of natural and ornamental plant hosts. It is likely that succulent, green plant hosts are more ubiquitous in the Southeastern U.S. than in arid regions like California, where green vegetation is generally confined to irrigated and managed land. Aggressive vineyard weed and alleyway management may therefore reduce sharpshooter breeding and feeding habitat, but several other problems related to the maintenance of soil structure and erosion prevention arise if the vineyard floor is barren of grasses.
An indirect way of managing Xf- positive vectors would be to limit the number of Xf-harboring plants near vineyards. The difficulty lies in determining the plants that are worth managing as (1) many plants that harbor Xf are asymptomatic; (2) Xf multiplies and spreads to different extents across species and results in some plants being “better” inoculum sources than others; and (3) insect vectors may not feed on all Xf-positive plants (Varela et al., 2001).
Application of insecticides, particularly systemic insecticides, from the time of vector detection in the spring is advised (Krewer et al., 2002). Glassy-winged sharpshooters often feed on the base rather than on the tips of canes, as well as on 2-year-old wood. Thus, early-season through mid- season management is particularly important, since early infections are more likely to lead to movement of bacteria to the cordon and trunk, where bacteria will not be removed through pruning practices (Feil et al., 2003). UGA Extension Circular 1151 (Hickey et al., 2018), Viticulture Management, is a poster offering a visual reminder and reference for sharpshooter scouting and management throughout the seasonal grapevine growth stages.


Testing
Grapevine tissues can be tested for PD infection using the polymerase chain reaction (PCR) method and enzyme-linked immunosorbent assay (ELISA). Using clean pruning shears, several petioles should be removed from vines showing PD symptoms. Many north Georgia vineyard owners can have their petioles tested for PD infection by bringing them to the Lumpkin or White county Extension offices. For a fee, the Plant Molecular Diagnostic Lab at the UGA Tifton campus can also test in-state or out-of-state samples. Those outside of Georgia should initially contact their local Extension agent or statewide small fruit or vineyard specialist for information about diagnosing PD on symptomatic vines, as other avenues for testing may be available.
Summary
Pierce’s disease (PD) may be the greatest threat to the growth and sustainability of wine grape industries in the Southeastern U.S. The first step to managing grapevine PD is understanding the threat of PD as dictated by the region, or sub-region, in which vines will be planted. It is highly advised that PD- tolerant cultivars be planted if a vineyard will be established in a region of high PD-threat. Growers should understand that there is a risk of planting Vitis vinifera and other PD-intolerant cultivars in several Southeastern U.S regions, including the mountain regions of northern Georgia and Piedmont regions in North Carolina. If PD-intolerant cultivars are planted, leafhopper vectors should be intensively scouted for and managed, and PD-infected vines should be immediately rogued out of the vineyard.
References
Anas, O., Harrison, U. J., Brannen, P. M., & Sutton, T. B. (2008). The effect of warming winter temperatures on the severity of Pierces disease in the Appalachian Mountains and Piedmont of the southeastern United States. Plant Health Progress, 9(1), 13. https://doi.org/10.1094/php-2008-0718-01-rs
Feil, H., & Purcell, A. H. (2001). Temperature-dependent growth and survival of Xylella fastidiosa in vitro and in potted grapevines. Plant Disease, 85(12), 1230–1234. https://doi.org/10.1094/pdis.2001.85.12.1230
Feil, H., Feil, W. S., & Purcell, A. H. (2003). Effects of date of inoculation on the within-plant movement of Xylella fastidiosa and persistence of Pierces disease within field grapevines. Phytopathology, 93(2), 244–251. https://doi.org/10.1094/phyto.2003.93.2.244
Hickey, C. C., Blaauw, B., Brannen, P., Pfeifer D., Nita, M., Hoffmann, M. (2022). Viticulture management (Publication No. C 1151). University of Georgia Extension. https://extension.uga.edu/publications/detail.html?number=C1151
Kamas, J., Stein, L., & Nesbitt, M. (2010). Pierce’s disease-tolerant grapes. Texas A&M AgriLife Extension Publication. https://aggie-horticulture.tamu.edu/wp-content/uploads/sites/6/2010/10/pd-grapes.pdf
Krell, R. K., Boyd, E. A., Nay, J. E., Park, Y., & Perring, T. M. (2007). Mechanical and insect transmission of Xylella fastidiosa to Vitis vinifera. Am J Enol Vitic., 58, 211–216. http://dx.doi.org/10.5344/ajev.2007.58.2.211
Krewer, G., Dutcher, J. D., & Chang, C. J. (2002). Imidacloprid insecticide slows development of Pierces disease in bunch grapes. Journal of Entomological Science, 37(1), 101–112. https://doi.org/10.18474/0749-8004-37.1.101
Lieth, J. H., Meyer, M. M., Yeo, K., & Kirkpatrick, B. C. (2011). Modeling cold curing of Pierces disease in Vitis vinifera ‘Pinot Noir’ and ‘Cabernet Sauvignon’ grapevines in California. Phytopathology, 101(12), 1492–1500. https://doi.org/10.1094/phyto-08-10-0207
Myers, A. L., Sutton, T. B., Abad, J. A., & Kennedy, G. G. (2007). Pierces disease of grapevines: Identification of the primary vectors in North Carolina. Phytopathology, 97(11), 1440–1450. https://doi.org/10.1094/phyto-97-11-1440
Redak, R. A., Purcell, A. H., Lopes, J. R. S., Blua, M. J., Mizell, R. F. III, & Anderson, P. C. (2004). The biology of xylem fluid-feeding insect vectors of Xylella fastidiosa and their relation to disease epidemiology. Annual Review of Entomology, 49, 243–270. https://doi.org/10.1146/annurev.ento.49.061802.123403
Smith, D. (2019, June 20). Pierce’s disease. Grape eXtension. https://grapes.extension.org/pierces-disease/
Varela, L. G., Smith, R. J., & Phillips, P. A. (2001). Pierce’s disease (Publication No. 21600). University of California Davis, UC ANR. http://iv.ucdavis.edu/files/24470.pdf
Wilcox, W. F., Gubler, W. D., & Uyemoto, J. K. (2015). Compendium of grape diseases, disorders, and pests (2nd ed.). APS Press.
Further reading:
Kamas, J. (Ed.). (n.d.). Pierce’s Disease Overview & Management Guide: A Resource for Grape Growers in Texas and Other Eastern U.S. Growing Regions. Texas A&M AgriLife Extension. https://aggie-horticulture.tamu.edu/wp-content/uploads/sites/6/2010/10/Texas-Grape-Growers-PD-Management-Guide.pdf
Varela, L. G., Smith, R. J., & Phillips, P. A. (2001). Pierce’s disease (Publication No. 21600). University of California Davis, UC ANR. http://iv.ucdavis.edu/files/24470.pdf
Status and Revision History
Published on Jul 15, 2019
Published with Minor Revisions on Aug 29, 2024


























































