The silverleaf whitefly (SLWF), Bemisia tabaci, (also known as sweet potato whitefly) is a pest of a wide variety of horticultural and agronomic crops in southern Georgia. Adults and nymphs (Figure 1) have piercing-sucking mouthparts and feed on phloem, the transport tissue of plants, and remove plant sap. While this direct feeding can damage plants and lead to additional problems with the accumulation of honeydew and sooty mold, whiteflies also inject salivary fluids while feeding, which can result in plant disorders and transmission of plant viruses. When viral pathogens are present, their transmission creates the greatest threat to the economical production of many vegetable crops, particularly tomatoes, snap beans, most cucurbit crops, and occasionally, cole crops. The potential for whitefly pest problems and viral disease incidence in Georgia varies greatly by year, location, and production season. Recent experience indicates that greater viral incidence can be observed when pest populations are high, even though few viruliferous (virus-carrying) whiteflies are needed to inoculate individual plants.


Photo: David Riley, University of Georgia, Bugwood.org
Viral disease incidence varies greatly from year to year; however, virus pressure is typically greatest in crops grown during the fall and is generally more severe in the areas around Tift County and Colquitt County in Georgia. Year-to-year variation often appears to be an “all or nothing” situation within affected areas in the fall—when the virus is present, it generally occurs at a very high incidence. Geographical distribution of virus incidence mirrors whitefly distribution within a year. As stated above, whitefly is a consistent pest during the fall in the areas around Tift and Colquitt counties, and populations have historically radiated various distances from this region within south-central Georgia. In contrast, in 2017, whiteflies were widely distributed throughout southern Georgia, and as a result, viral diseases were widespread in tomatoes throughout the area.
Virus Transmission
Whiteflies transmit viruses in either a semi-persistent or persistent manner (Figure 2). The method of transmission is important as it provides clues about the timeframes involved in virus acquisition and inoculation and the likely impact of potential management practices. Both semi-persistent and persistent viruses require the insect to feed on the phloem for an extended period, usually a minimum of multiple minutes. The insects do not acquire or inoculate (transmit) these viruses by simply probing epidermal cells. The time required to acquire the virus from an infected plant is known as the “acquisition access period,” while the time required to introduce the virus to a healthy plant is called the “inoculation access period.”
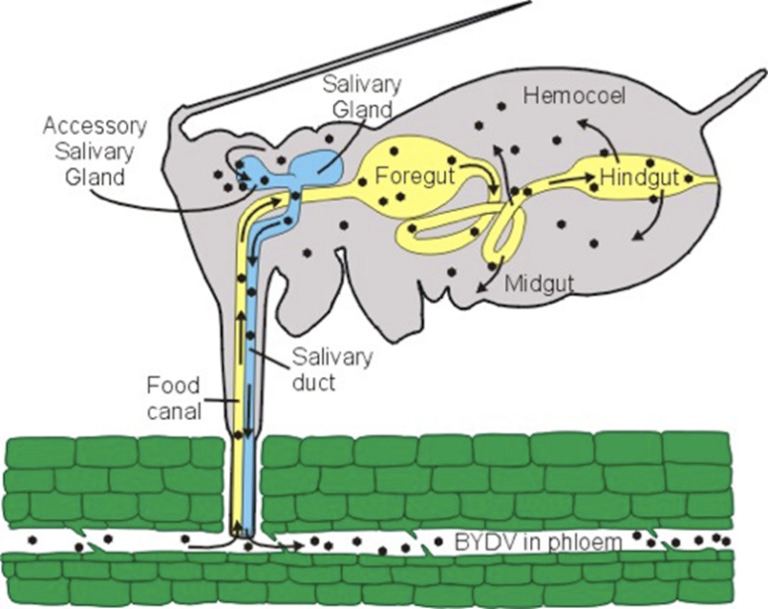
Photo: D’Arcy and Domier, 2000. Plant Health Instructor, DOI 10.1094/PHI-I-2000-1103-01, American Phytopathological Society
With semi-persistent viruses, the virus enters the insect’s foregut (the anterior part of the gut, toward the mouth), is inoculated to uninfected plants when the insect feeds, and is lost within a few hours after acquisition. The insect loses the ability to transmit the virus and must feed on another infected plant before it can once again transmit the virus. With persistent viruses, the virus must enter the insect’s hemolymph (the fluid equivalent to blood in invertebrates) via the midgut and migrates to the salivary glands before the insect can inoculate the virus. This delay in the ability to inoculate the virus is known as the latent period. With semi-persistent viruses there is no latent period—they can inoculate the virus immediately after acquisition—while the latent period may be hours or days for persistent viruses. With persistent viruses, insects maintain the ability to transmit the virus for extended periods and may remain viruliferous throughout their life. The acquisition and latent periods require time, which provides an opportunity for insecticides to kill the whiteflies, or impact their feeding, reducing virus inoculation. For this reason, persistent viruses have a better potential for management with insecticides, but they also produce insects that remain viruliferous for longer periods. A list of common whitefly-transmitted viruses that affect vegetable crops in Georgia with their mode of transmission is provided in Table 1.
Table 1. List of common whitefly-transmitted viruses on vegetables with their mode of transmission in Georgia.
|
Virus species |
Genus |
Mode of transmission |
Host |
|
Cucurbit leaf crumple virus |
Begomovirus |
Persistent-circulative |
Cucurbitaceous crops and snap beans |
|
Cucurbit yellow stunting disorder virus |
Crinivirus |
Semi-persistent |
Cucurbitaceous crops |
|
Squash vein yellowing virus |
Ipomovirus |
Semi-persistent |
Cucurbitaceous crops |
|
Tomato yellow leaf curl virus |
Begomovirus |
Persistent-circulative |
Solanaceous crops |
|
Tomato chlorosis virus |
Crinivirus |
Semi-persistent |
Solanaceous crops |
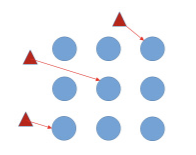
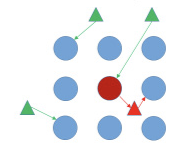
The management of viruses is greatly affected by the importance of primary and secondary infection (Figure 3). Primary infection occurs when an insect already carrying the virus enters the field and infects an uninfected plant. This requires a short period of time (minutes), and insecticides generally show minor impacts on the spread of the virus within a field unless whiteflies are at low densities. Secondary infection occurs when a virus-free whitefly lands on an infected plant within the field, feeds on that plant and acquires the virus, then moves to an uninfected plant and feeds long enough to inoculate that plant. Secondary infection may require an extended period of time and can therefore be suppressed by insecticides. However, when SLWF populations are high, insecticides may be ineffective at mitigating secondary infection.
Primary Spread
Virulifierous whiteflies enter a field and inoculate non-infected plants
Secondary Spread
Non-virulifierous whiteflies enter a field and acquire the virus from infected plants and inoculate non-infected plants
The major viruses transmitted by whiteflies in southern Georgia include cucurbit leaf crumple virus, cucurbit yellow stunting disorder virus, and tomato yellow leaf curl virus. Additionally, squash vein yellowing virus poses a potential threat in Georgia, as it occurs in neighboring Florida. Whiteflies do transmit other viruses within vegetable crops, but they have not presented significant problems in Georgia to date. All of these viruses are phloem-limited and are not known to be seed-transmitted. Mechanical transmission has not been reported either. The primary mode of transmission seems to be vector-mediated.
Cucurbit leaf crumple virus
Cucurbit leaf crumple virus (CuLCrV; family Geminiviridae, genus Begomovirus) can infect snap beans and cucurbits, including cucumber, cantaloupe, squash (yellow, zucchini, and winter squash), pumpkin, and watermelon. CuLCrV was first reported in the Western United States (Arizona, Texas and California) and Northern Mexico. In the Southeastern U.S., CuLCrV was first reported in squash in north-central and northeastern Florida in 2006. In 2016 and 2017, outbreaks of SLWF and CuLCrV in the Southeastern U.S. caused extensive damage to cucurbits and snap beans.


Symptoms: Symptoms of CuLCrV include leaf curling and crumpling, chlorotic (abnormally pale due to insufficient chlorophyll) leaf spots, and interveinal yellowing. Stunting and growth distortion are common when plants are severely infected and whitefly pressure is substantially high. Symptoms may also vary among different cucurbit hosts. For example, in yellow straightneck squash, leaves may be curled with rounded edges (Figure 4), while marginal leaf distortion may be minimal in CuLCrV-infected zucchini. Misshapen fruit with green streaks can be observed in yellow straightneck squash whereas obvious fruit symptoms may not be seen in zucchini. In both hosts, thickening and distortion of foliage is commonly observed (Figure 4). The virus can also infect snap beans and, in some cases, symptoms are more severe than observed in cucurbits. Severe leaf distortion, rugosity (surface roughness), and stunting can be seen in snap beans, and plants infected early in development frequently die (Figure 5).


Mode of transmission: CuLCrV is transmitted in a persistent and circulative manner by SLWF with an acquisition period of approximately 30 minutes and a latent period of 6-8 hours. The inoculation period is likely in the range of 30 min or less. The viruliferous whitefly can continue to transmit CuLCrV for several days.
Cucurbit yellow stunting disorder virus
Cucurbit yellow stunting disorder virus (CYSDV; family Closteroviridae, genus Crinivirus) is only known to infect cucurbits including watermelon, cantaloupe, cucumber, and squash. CYSDV is also known to infect pigweed in the Southeastern U.S.


Symptoms: The symptoms on cucumbers and cantaloupe show severe yellowing that starts as an interveinal mottle especially observed on older leaves (Figure 6). Visual symptoms may mimic a magnesium deficiency in cantaloupe. As the disease progresses, severe mottling and yellowing of foliage may be observed. Yellowing becomes more severe as leaves grow older. Yellowing symptoms can be confused with nutritional disorders, issues with soil pH, micronutrient deficiencies, natural senescence (aging), and pesticide phytotoxicity (toxic damage). Visual diagnosis of CYSDV is a challenge for growers, Extension agents, and researchers. For confirmation, diagnostic testing should be performed by a reputable laboratory. Severely infected plants display stunting, hence the name “yellow stunting disorder.” More severe symptoms can be observed when CYSDV and CuLCrV occur together (Figure 6), which is more often the case than either alone.
Mode of transmission: CYSDV is transmitted in a semi-persistent manner by SLWF. Acquisition and inoculation periods are less than 2 hours each. Thus, adults can acquire and transmit the virus in less than 4 hours. In controlled studies, it was observed that CYSDV can persist in the vector for 7 to 9 days with a half-life of 3 days.
Tomato yellow leaf curl virus
Tomato yellow leaf curl virus (TYLCV; family Geminiviridae, genus Begomovirus) affects mainly tomato in the southeastern U.S. In addition to tomato, other known hosts for TYLCV are pepper, and lisianthus. Several weed species may also carry TYLCV as symptomatic or asymptomatic hosts.
Symptoms: The symptoms of TYLCV include severe stunting of shoots, resulting in a bushy appearance. Distinct symptoms on leaves include interveinal chlorosis and upward and inward rolling of the leaf margins (Figure 7). Plants infected early in development are also stunted and can be easily distinguished from non-infected plants. Fruit set can be severely affected if infection occurs in or prior to the flowering growth stage. Symptoms on fruit are not distinct, but infected plants produce mottled and misshapen fruit.


Mode of transmission: TYLCV is transmitted in a persistent and circulative manner by SLWF. The acquisition and inoculation periods are 20 and 10 minutes, respectively. TYLCV has a 20- to 24-hour latent period, which provides the opportunity for insecticides to impact secondary spread. In some cases, TYLCV can also be transmitted within whiteflies both sexually (from male to female and female to male) and transovarially (from female to eggs).
Squash vein yellowing virus
Squash vein yellowing virus (SqVYV; family Potyviridae, genus Ipomovirus) causes watermelon vine decline (WVD). In Georgia, this disease and the associated virus has been rare, but there is a risk of future outbreaks as the disease regularly occurs in Florida.
Symptoms: As the name suggests, the symptoms of WVD include the sudden decline and death of vines (Figure 8). This can occur either prior to harvest or at harvest. Fruit symptoms often include rind necrosis (premature cell death) and the degradation of internal flesh. When fruit infection is extreme, internal flesh may appear leathery with a dark-red, glistening appearance. Cucurbits have been confirmed as the preferred host for the virus, but the most striking symptoms have only been seen in watermelon.
In Florida, balsam apple (Momordica charantia), a cucurbit weed, was found to be a common reservoir host for SqVYV.


Mode of transmission: SqVYV is transmitted in a semi-persistent manner by SLWF. Acquisition and inoculation periods are less than 4 hours each. Adults were shown to acquire and transmit the virus in a minimum of 3 hours, but they remained viruliferous for only 24 hours.
Cucurbit chlorotic yellows virus
Cucurbit chlorotic yellows virus (CCYV; family Closteroviridae genus Crinivirus) is part of an emerging complex of whitefly-transmitted viruses associated with cucurbit yellows disease. CCYV was first reported in Japan in 2004, causing significant losses in melon production, and has since spread globally. CCYV transmission occurs in a semipersistent manner by B. tabaci MEAM1 and MED. Early infection of cucumber, melon, and watermelon plants by CCYV results in reduced yields and reduced sugar content in melons. In the United States, CCYV emerged as an important pathogen affecting cucurbit crops, particularly in melons and watermelons, following its introduction to the Southwestern region in 2014. In the Southeast, CCYV was first identified in Georgia in 2021 and has been found infecting cucurbits such as squash, melon, watermelon, and cucumber. Wild radish also has been identified as a host of CCYV in cooler months when cucurbits are not grown.
Symptoms: The symptoms of CCYV are nearly indistinguishable from that produced by CYSDV. Symptoms start as chlorotic spots with diffuse margins, which later coalesce and develop into interveinal chlorosis (Figure 9). On an infected plant, older leaves appear brittle with interveinal chlorotic spots, while younger leaves are also chlorotic with yellowing between the veins (Figure 9). CCYV infections can easily get confused with those caused by various nutritional deficiencies.

Tomato chlorosis virus
Tomato chlorosis virus (ToCV; family Closteroviridae, genus Crinivirus) has a genome comprised of a single-stranded positive-sense RNA (ssRNA) divided between two segments, designated as RNA1 and RNA2. The virus is transmitted by whiteflies (B. tabaci; family: Aleyrodidae) in a semipersistent manner. Initially reported in Georgia in 2011, recent surveys indicate its widespread occurrences in tomato-growing regions. ToCV has a broad host range, infecting crops such as tomato, cowpea, sweet pepper, and tomatillo, in addition to several weeds and ornamental plants. Recently, ToCV was found infecting tomatillo (Physalis philadelphica L.) in Georgia.
Symptoms: Typical symptoms of ToCV include leaf thickening, leaf rolling, interveinal chlorosis, marginal chlorosis, and a brittle texture, particularly noticeable in the lower canopy of the plant (Figure 10). While some of these symptoms overlap with those caused by TYLCV, ToCV symptoms tend to be more pronounced on the lower leaves, whereas TYLCV symptoms are more prominent on the upper leaves (Figure 10). These symptoms frequently are misidentified as abiotic stress factors, such as nutritional deficiencies or heat stress.

Management of Vector and Viruses
The production season and corresponding planting date has a tremendous impact on potential virus incidence in Georgia. In typical years, both whitefly and virus incidence are difficult to detect or undetectable during the spring production season and may not require any specific management practices; however, whitefly and virus pressure can easily overwhelm most management strategies in the fall.
Whenever possible, the management of plant viruses is best achieved with resistant varieties of the host plant. The primary method of managing TYLCV is through the use of resistant tomato varieties (Figure 11). Currently, these varieties possess semi-dominant resistance genes (Ty-1 or Ty-3). Resistant cultivars are not immune to viral infection, and they can show mild symptoms and serve as a reservoir for the virus. Unfortunately, as of 2018, resistant varieties are not commercially available for the other whitefly-transmitted viruses addressed in this publication.

As for most pests, always ensure that transplants are free of both viruses and vectors before planting. Transplants should be produced in virus-free locations when possible. Avoid planting adjacent to older crops that are already infested with whiteflies and/or any of these viruses.
Other management approaches may suppress or delay virus incidence when disease and vector pressure is low, but there is minimal to no impact when whitefly density and virus incidence are high. Alternative management strategies, such as the use of reflective mulches and insecticidal control of the vector, have also been shown to work best when implemented in a coordinated combination of approaches.
Managing viruses through insecticidal control of the whiteflies will work best when vector densities are low and secondary infection is the primary method of virus spread. Insecticides currently used against whiteflies are efficacious on immature insects but provide little or no impact on adults, so they provide little impact in rescue situations. When whitefly densities are moderate to high and they are entering the field already carrying the virus (primary infection), insecticides have shown little or no impact on virus incidence.
Sanitation is important, particularly in preventing carryover and increases in whitefly populations and virus incidence from one crop to subsequent crops. Growers should remove and destroy old crops/volunteers (e.g., plowing/physical removal) as soon as a crop is finished. Removing weeds in and around the fields can be beneficial, as they may be reservoirs for the virus and support whitefly populations.
References
Adkins, S., Polston, J. E., & Turechek, W. W. (2009). Cucurbit leaf crumple virus identified in common bean in Florida. Plant Dis., 93, 320. https://doi.org/10.1094/PDIS-93-3-0320B
Adkins, S., Webb, S. E., Achor, D., Roberts, P. D., & Baker, C. A. (2007). Identification and characterization of a novel whitefly-transmitted member of the family potyviridae isolated from cucurbits in Florida. Phytopathology, 97, 145–54. https://doi.org/10.1094/PHYTO-97-2-0145
Berdiales, B., Bernal, J. J., Saez, E., Woudt, B., Beitia, F., & Rodriguez-Cerezo, E. (1999). Occurrence of cucurbit yellow stunting disorder virus (CYSDV) and beet pseudo-yellow virus in cucurbit crops in Spain and transmission of CYSDV by two biotypes of Bemisia tabaci. Eur. J. Plant Pathol., 105, 211–215. https://doi.org/10.1023/A:1008713629768
Martini, X., Paret, M., Polston, J. E., Adkins, S., Roberts, P., Nyoike, T., & Liburd, O. E. (2022). Recommendations for management of whiteflies, whitefly-transmitted viruses, and insecticide resistance for production of cucurbit crops in Florida (Publication #ENY-478). University of Florida IFAS Extension. https://doi.org/10.32473/edis-in871-2022
Momol, T., Olson, S., Funderburk, J., & Sprenkel, R. (2001). Management of Tomato yellow leaf curl virus (TYLCV) in tomato in North Florida. University of Florida IFAS. http://edis.ifas.ufl.edu/pdffiles/NF/NFREC100.pdf
Sánchez-Campos, S., Rodríguez-Negrete, E. A., Cruzado, L. Grande-Pérez, A., Bejarano, E. R., Navas-Castillo, J., & Morione, E. (2016). Tomato yellow leaf curl virus: No evidence for replication in the insect vector Bemisia tabaci. Scientific Reports, 6, 30942. https://doi.org/10.1038/srep30942
University of California Agriculture and Natural Resources (2008). UC Pest Management Guidelines: Cucurbits, Cucurbit Yellow Stunting Disorder, Pathogen: Cucurbit yellow stunting disorder virus (CYSDV). http://ipm.ucanr.edu/PMG/r116100211.html
Webb, S., Adkins, S., & Reitz, S. (2012). Semipersistent whitefly transmission of Squash vein yellowing virus, causal agent of viral watermelon vine decline. Plant Dis., 96, 839–844. https://doi.org/10.1094/PDIS-09-11-0761
Webb, S. E., Liburd, E. O., Nyoike, T. W., Akad, F., & Polston, J. E. (2017). Whitefly-transmitted cucurbit leaf crumple virus in Florida. IFAS Extension, University of Florida. https://edis.ifas.ufl.edu/pdffiles/IN/IN71600.pdf
Wisler, G. C., Duffus, J. E., Liu, H. Y., & Li, R. H. (1998). Ecology and epidemiology of whitefly-transmitted closteroviruses. Plant Dis., 82, 270–280. https://doi.org/10.1094/PDIS.1998.82.3.270
Status and Revision History
Published on Oct 22, 2018
Published with Minor Revisions on Mar 19, 2024


























































