In the spring of 1953, Watson and Crick published a one-page letter in Nature, one of the world’s most recognized scientific journals. The letter explained they had discovered the three-dimensional structure of deoxyribonucleic acid (DNA). This letter also contained what is arguably the most famous understatement in scientific history: “This structure (DNA) has novel features which are of considerable biological interest.” Some 30 years later, Alec Jefferys developed a process for “genetic fingerprinting,” a process for identifying individuals based on their unique genetic code. Nine years after Jefferys’ discovery, genetic fingerprinting was thrust into the limelight during the O.J. Simpson murder trial. Right or wrong, in today’s world, genetic testing is considered the strongest form of evidence in every situation from criminal trials to cancer screening to paternity testing. This has opened the door to innumerable applications of DNA-based technology to genetic improvement of livestock species.
In the early 1980s, Soller and Beckmann described the possible uses of DNA-based technology in livestock breeding, and surprisingly, their vision was not much different than the way DNA is used today in the livestock industry. They suggested that DNA-based technology would be beneficial in constructing more precise genetic relationships to be used in genetic evaluation programs, followed by parentage determination, and the direct identification of genes that affect a trait. Genomic testing is not necessarily a new idea in the eyes of science, but its application in today’s beef industry is still met with some confusion. Much of this confusion is related to terminology. Genetic terminology can be intimidating at first, but with some study it can be mastered. Most companies marketing genetic testing technology will help customers interpret the results from their respective tests, and Extension agents and specialists have access to a great deal of information on genetic technologies. Spangler and Schiermiester (2014) provide a list and definitions of common terms used in DNA testing. The objective of this bulletin is to provide a clear and concise reference for genomic testing currently available for beef cattle producers.
Testing for parentage or simply-inherited traits
Genetic testing is based on the principles of inheritance. Inheritance is most easily explained using “simply inherited” traits. Simply inherited traits are controlled by one or a few genes. For cattlemen, color and horned status are the best examples. Many producers will remember when almost every conversation about a potential herd sire involved the words “homozygous black and homozygous polled.” As an example, the black color status in Angus is controlled by a single gene with two possible outcomes: black calf or red calf. The outcome is dependent upon which two alleles (versions of the gene) the animal inherits from its sire and dam. Each animal inherits one allele from the sire and one allele DNA from the dam. In the case of coat color, the black allele is dominant to red. This means that a black animal may carry two black alleles or one black and one red allele. Because the red allele is recessive, an animal must possess two copies of the red allele for the animal to actually be red in color. A similar situation exists for horned status where the horned animal carries two copies of the recessive allele.
The idea of parents donating a copy of each allele to the offspring is the basis for parentage testing in cattle. Parentage testing has been available for a while, but it is extremely useful for producers who expose cows to multiple sires or employ a cleanup bull after artificial insemination (AI) exposure. Genotyping (investigating the genetic constitution) animals allow for the true sire to be correctly identified. These genetic tests promote informed breeding and culling decisions by helping to identify bulls that are making the largest amount of progress toward the producer’s genetic goals. Unlike simply inherited traits, parentage tests look at many genes or DNA markers to compare calf to parent with greater certainty (Figure 1).
Let’s say we have a calf with two potential sires.
For simplicity’s sake, we will examine only one gene.
| Calf | Dam | Sire 1 | Sire 2 | ||||
|---|---|---|---|---|---|---|---|
| Gene 1 | Gene 1 | Gene 1 | Gene 1 | ||||
| Allele 1 | Allele 2 | Allele 1 | Allele 2 | Allele 1 | Allele 2 | Allele 1 | Allele 2 |
| A | B | B | B | A | B | B | B |
We can see by the presence of the A allele or “marker” in the calf that sire 1 must be the calf’s true sire. There is no possible way for a mating between the dam and sire 2 to result in a calf that is a carrier of the A allele.
Genomic tests
Recently, genomic testing for beef cattle has evolved to include “high-throughput” testing, meaning that thousands of markers (or single nucleotide polymorphisms, or SNPs) are read from an animal’s DNA. Producers need only to submit a blood, hair, or tissue sample from an animal to the breed association (Figure 2). Please note that hair samples must be pulled and have the root attached so that DNA can be extracted. Cutting hairs will not work. The switch of the tail is the recommended location for extracting hair samples. After collection and submission to the respective breed association, samples are submitted to the genotyping laboratory where several thousand markers are read from the DNA extracted from the samples. The most common genomic test available for cattle reads around 50,000 DNA markers or SNPs using a technology embedded in a small chip called a SNP chip (Figure 3). The SNPs are changes in the DNA that can be responsible for genetic variation among animals. Although in most cases SNPs are not inside the sequences of DNA that we know as genes, they can be linked to genes to give us some information about them.

Figure 2. Blood and hair samples for genomic testing. Photo courtesy of Angus Genetics Inc.

Figure 3. SNP chip from Illumina Inc. able to test eight animals at the same time.
Having the ability to read thousands of SNPs is a tremendous advantage for producers because most economically important traits like calving ease, dry matter intake, feed efficiency, hot carcass weight, marbling score, and tenderness are controlled by many genes as opposed to a single gene like hide color or horned status. This means that many modern tests can make predictions about the genetic merit of an animal more precisely and at a younger age than traditional expected progeny difference (EPD). This is not to say that producers should ignore phenotype. An animal’s performance is still a good measure of genetic potential, and not every SNP involved in the expression of desirable (or undesirable) traits has been found. Speaking generally, however, more information about an animal’s genetic makeup offers a better assessment of its genetic potential and therein lies the value of genetic testing.
When genomics was first adopted by the beef industry, results of genomic testing were reported as molecular breeding values (MBV). MBV is simply the sum of effects of each SNP an animal possesses. Figure 4 shows a very simple example of how to calculate MBV, supposing an animal was tested for only three SNPs. After the SNP effects are calculated, we add the effects over both alleles of each SNP. In this example, allele A from SNP 1 has a value of 1; as the animal received the allele A from both parents, it will be counted twice. The same happens for each SNP, until the effects of all SNP are added up. The MBV for this animal will be 1+1- 0.3+0.3+0.5+0.5 = 3.0.
In the early days of genomic testing, MBVs were reported as separate values. Later, the MBV was combined with EPD into a genomic-enhanced EPD (GE-EPD). This combination was necessary because the thousands of markers that are read from the DNA do not explain all of the genetic variability of the traits; therefore, this missing portion can be obtained from EPD because the EPD represents pedigree and performance. In addition, the gain in accuracy that is promised by using genomics comes from the fact that one extra piece of information is being used to estimate the genetic merit of the animal. At the end of the day, accuracy is all about the amount of information used for the estimation of EPD.
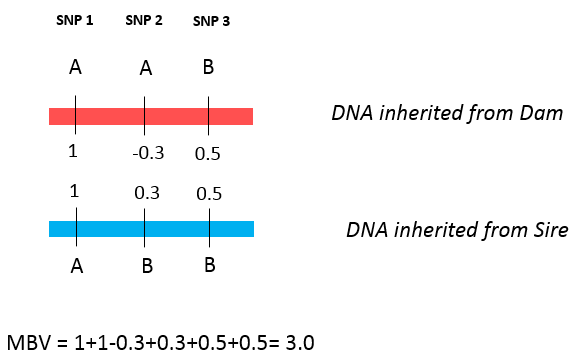
Figure 4. The calculation of MBV using a small example of three SNPs. A and B are alleles from each SNP. The red and blue DNA strands were inherited from the dam and sire, respectively.
In 2017, the beef industry began moving to an improved method using SNPs for genomic testing. This new way is called “single-step,” short for “single-step genomic best linear unbiased predictor” or “ssGBLUP.” Before ssGBLUP, SNP effects, MBV, and GE-EPD were predicted in successive steps. Now, in single-step, SNP information is added directly to the evaluation system for the calculation of EPD (Figure 5). Additionally, in single-step, SNP are used to compute better relationships among individuals. Relationships obtained through pedigrees are expected values, meaning that the proportion of genes shared between an animal and its grandsire, for example, is expected to be 0.25. When we have both the animal and its grandsire genotyped, we can calculate the actual proportion of SNP that they share (e.g., 0.31 instead of 0.25). Having real as opposed to expected relationships gives more precise information for genetic evaluations, because we know the correct amount of DNA is being inherited. Several studies in beef cattle have shown that single-step gives more accurate GE-EPD than the multistep methods (Lourenco et al., 2015).
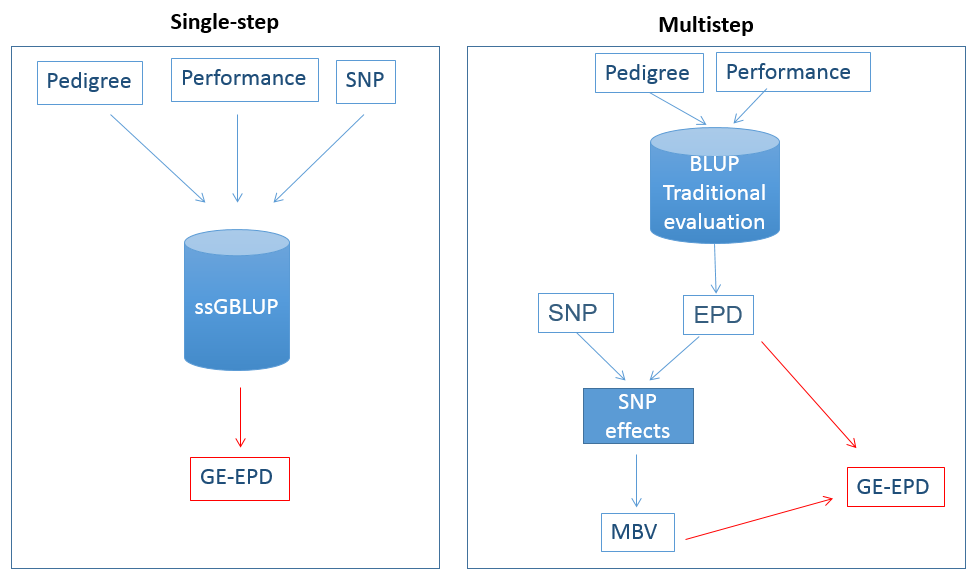
Figure 5. A comparison between the single-step method and the multistep method,the first method implemented by the beef cattle industry for genomic testing. The blue cylinder “BLUP traditional evaluation” represents the classical evaluation that uses only pedigree and performance records; the blue cylinder “ssGBLUP” represents the single-step that uses pedigree, performance, and SNP at the same time.
In single-step, all pedigree information, performance records, and SNP are included in a single evaluation that gives only one output: GE-EPD. Although it seems complicated, especially because it involves a lot of heavy math, the machinery of single-step can be translated into something easier. A pickup truck requires gas and an engine oil to run; if a fuel additive is aggregated, the truck can run even better. The pickup truck can be replaced by the single-step machinery, the gas by the performance records, the engine oil by the pedigree, and the fuel additive by the SNPs. In the same way the fuel additive boosts the truck performance, the SNP genotypes improve the performance of the evaluation machinery.
Because not all animals in the pedigree are genotyped, single-step also has the ability to compute better relationships for nongenotyped animals if they are related to genotyped animals through the pedigree. Single- step started being developed in 2009 by the Animal Breeding and Genetics group at the University of Georgia. Since then it has been adopted and widely used by some of the biggest names in the U.S. livestock genetics industry. The American Angus Association and Angus Genetics Inc. started releasing GE-EPD from single- step in July of 2017. In their first single-step evaluation for growth traits, records on birth and weaning weight were available for over 8 million animals and postweaning gain were available for over 4 million. In addition, pedigree information was available for over 10 million animals; among those, 350,000 were genotyped for nearly 50,000 SNP.
Santa Gertrudis implemented the single-step protocol for calculating GE-EPDs around 2015. Other breeds have also developed GE-EPDs: Hereford, Simmental, Red Angus, Gelbvieh, Limousin, Charolais, Shorthorn, and Brangus, although these breeds may use different mathematical techniques to incorporate SNP information into evaluations.
Accuracy and GE-EPDs
The big gamble with using EPDs as a fail-safe selection tool is the accuracy of the prediction in young cattle that have few, if any, progeny. The lack of confidence associated with EPDs on young cattle comes from not having progeny or performance data, both of which increase the accuracy of the EPD. In young bulls, for example, most of their genetic value is based on their pedigree. As these animals age and have offspring, we know more and more about their genetic merit. This increased confidence is denoted by an increase in the accuracy value (0–1 scale) associated with each EPD. It does not necessarily mean that the EPD increases if accuracy increases. It just means the EPD becomes closer to the true value, whether it increases or decreases. Remember that EPD stands for expected progeny difference. Genotyping a young animal increases accuracy because SNP genotypes have similar value to adding the performance of five to 50 progeny, depending on the trait that is being evaluated. Samples of DNA can be collected very early in life, and unlike metabolism or hormones, the genetic code does not change over the course of an animal’s life. Genomic testing allows producers to take a virtual snapshot of a portion of the genes that are flowing in the population and that regulate economically important traits. This increased knowledge of the genomics of an animal allows for increased confidence in the GE-EPD.
Summary
Using genomic testing in beef cattle operations allows early prediction of genetic merit and increases the value of young breeding stock. Some of the traits of interest are expensive to measure in the live animal (e.g. reproductive traits, feed efficiency, or tenderness). For seedstock producers, genomics are the way of the future, and the adoption of this technology has already begun to determine success in the market. Commercial cattlemen need to evaluate cost vs. benefit. The cost of using this technology is decreasing as time passes, but it is important for producers to analyze the value of this data before employing this tool to ensure that it is economically justifiable.
For more information on genetic testing, contact your local Extension office at 1-800-ASK-UGA-1 or extension.uga.edu.
Glossary of Terms
accuracy value – numerical value ranging from 0 to 1 associated with an EPD to give the reader a confidence level associated the EPD
allele – one of two or more alternative versions of a gene
deoxyribonucleic acid (DNA) – a self-replicating compound present in almost all living things that carries the basic genetic code from one generation to the next
expected progeny differences (EPD) – statistical values that represent the genetic evaluation of an animal as a parent using information taken for the pedigree, individual performance and performance of progeny
gene – a specific sequence of DNA that codes for the construction of a protein which causes an organism to express a heritable trait
genetics – the study of heredity and inheritance of characteristics across biological generations
genetically enhanced expected progeny differences (GE-EPD) – EPDs that include information taken from the results of genomic testing; will typically have higher accuracies than EPDs for animals of the same age that have not been genomically tested
genome – the complete set of genetic information in a cell or organism
genomics – a branch of molecular biology concerned with the structure, function, evolution and mapping of genomes
heterozygous – describes an organism has two different copies of an allele for a trait; one copy donated from each parent
homozygous – describes an organism has two copies of the same allele; one copy donated from each parent
molecular breeding value (MBV) – the sum of the effect of each of the individual SNPs contained in a specific organism
nucleotide – the basic structural unit of nucleic acids such as DNA
polygenic traits – physical traits controlled by a large number of genes; examples include marbling, docility, and weight gain
simply-inherited traits – physical traits controlled by a small number of genes, usually one; examples include horned status and hide color
single nucleotide polymorphism (SNP) – DNA sequence variations that occur when a single nucleotide in a genome is altered
References
Lourenco, D. A. L., Tsuruta, S., Fragomeni, B. O., Masuda, Y., Aguilar, I., Legarra, A., Bertrand, J. K., Amen, T. S., Wang, L., Moser, D. W., & Misztal, I. (2015). Genetic evaluation using single-step genomic best linear unbiased predictor in American Angus. J. Anim. Sci., 93, 2653–2662.
Soller, M., & Beckmann, J. S. (1983). Genetic polymorphism in varietal identification and genetic improvement. Theor. Appl. Genet., 67, 25–33.
Spangler, M. L., & Schiermiester, L. (2014). DNA Tests for the Genetic Improvement of Beef Cattle. NebGuide G1856. University of Nebraska, Lincoln.
Status and Revision History
Published on Mar 04, 2019
Published with Full Review on Aug 18, 2022


























































