In the Southeastern U.S., beef cattle producers focus on forage management and maximizing the grazing season. To that end, Southeastern cattle spend the vast majority of their lives either grazing or consuming stored forage. Cereal grains and coproduct feeds are also commonly used nutritional supplements for cattle in times of elevated nutrient requirement. Any disease or disorder in the beef herd represents a nutrient drain and subsequent economic inefficiency. Sudden shifts in dietary composition as well as mineral imbalances are the primary causes for most nutritional and metabolic disorders. This publication contains an overview of the common nutritional and metabolic disorders that may affect beef herds in the Southeast along with management strategies that can be used to minimize the effects of these disorders in beef cattle production systems.
Bloat
For most producers, bloat is the most recognizable nutritional disorder. Bloat is a buildup of gas in the rumen, which is the large fermentation chamber that makes up the majority of the foregut in cattle. In its simplest form, gas production from the fermentation of feed particles exceeds the amount of gas lost through eructation or belching. If rumen gases continue to be produced in excess, the rumen distends, producing a “ballooned” look on the animal’s left side (Figure 1). Continued accumulation of ruminal gases will eventually compress the diaphragm, impede expansion of the lungs, and cause asphyxiation. Producers should watch for rapid abdominal distention and other signs of discomfort such as foot stomping or kicking at the belly.

Bloat susceptibility is both animal- and environment-driven. Like humans, some animals have a more sensitive stomach and are more prone to bloat. Typically, these animals are diagnosed early and labeled as “chronic bloaters” because they tend to bloat daily. Chronic bloaters are typically marketed early through a sale barn to minimize losses in performance. Environmentally driven bloat can happen because of sudden changes in the diet of fed animals or as a result of an accident wherein a large amount of feed is spilled in an area where animals will have access to it, such as when creep feeders are damaged (Gadberry & Powell, 2011).
Pasture or legume bloat is also a possibility. Legume bloat is caused by high levels of soluble protein in the plant that increases the viscosity of the animal’s rumen fluid. This causes fermentative gases to become trapped in stable foam as opposed to a single bubble or loose froth, which is more typically seen with other types of bloat. Several species of forage crops including alfalfa, ladino or white clover, and Persian clover can cause legume bloat if cattle are not managed properly (Ball et al., 2015). Other legume species including (but not limited to) arrowleaf and berseem clover, crownvetch, sericia and annual lespedeza, and birdsfoot trefoil contain elevated levels of tannin in the leaves that cause soluble protein to precipitate in the rumen, and thus pose minimal risk for legume bloat (Ball et al., 2015). Additionally, tropical legumes such as perennial peanut, cowpea, and kudzu rarely cause bloat. Although pasture bloat is primarily associated with the aforementioned legumes, it is important to note that cattle can experience bloat on lush ryegrass or small grains pasture, typically in spring. These plants are high in soluble protein and rapidly growing, so animals that overeat can be at risk.
Prevention:
Preventing bloat is based on management rather than drugs or vaccines. Cattle that have been undernourished for any length of time should not be turned out onto lush legume pastures or given access to large quantities of supplemental feed. Cattle should be filled up on hay so that overconsumption of feeds that may cause bloat is less likely. Feeding Rumensin® has been shown to reduce the incidence of bloat in grain-based diets, and poloxalene, offered by trade names Therabloat, Bloat Guard, Purina Saf-T-Block, or Sweetlix Bloat Guard, is a compound that has been shown to break up the stable gases that form in the rumen and cause bloat. Poloxalene can be top-dressed onto supplement at a rate of 2 grams per 100 pounds of body weight per head per day. Commercial poloxalene blocks are also available. These are typically salt-molasses blocks that contain poloxalene at a rate of 30 grams of poloxalene per pound. It is important that cattle be given access to these blocks for at least three days before allowing them access to lush legume pasture.
Treatment:
A gastroesophageal tube may be used to relieve pressure in bloated animals, and a ruminal infusion of poloxalene may be added through the tube to break down the stable gas, allowing the animal to belch. A speculum may be used to ease passage of the tube through the mouth and prevent the animal biting and restricting flow of gas or liquid through the tube. It is important not to simply drench bloated cattle. Without the protection of the stomach tube, the danger of inhalation is too great (Gadberry & Powell, 2011). Animals can suffocate or develop pneumonia due to inhalation of liquid into the lungs.
In the most severe bloat cases, a trocar may be used to puncture the body and ruminal wall on the animal’s left side. This should be reserved as a last resort because of the high risk of infection. Animal performance is often decreased by prolonged oxygenation of the rumen environment.
Ruminal Acidosis Complex
As indicated by its name, acidosis is a condition that results from a decrease in ruminal pH and is usually associated with a rapid dietary shift from a high roughage to a high concentrate diet. Acidosis is problem usually discussed in the context of feedlot cattle, but there is a moderate incidence in the Southeast. These cases are most often observed in cattle that are being fed for rapid gain such as may be found in feedlots, performance tests, and 4-H steer or heifer projects. Also, in times of hay shortage, poorly formulated hay replacement rations can lead to increased incidence of acidosis in the cow herd.
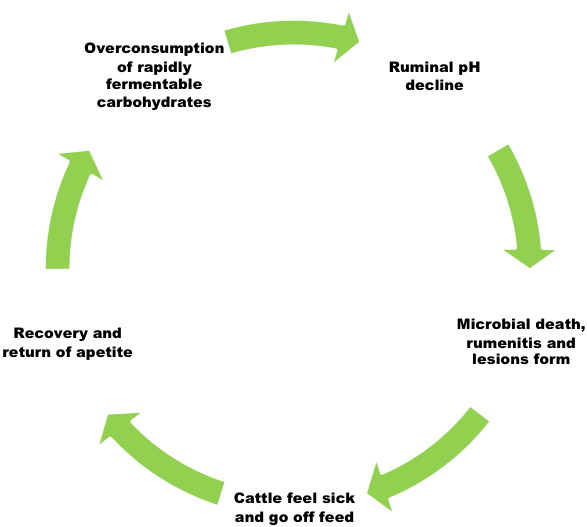
The bacterial population of the rumen is extremely diverse. Some species of bacteria specialize in the fermentation of fiber (structural carbohydrates)and some specialize in the fermentation of starch (storage carbohydrates). All bacteria produce volatile fatty acids as a fermentative by-product. These acids are the basis of energy metabolism in ruminants. If these acids are produced more rapidly than they can be absorbed, they accumulate in the rumen and cause the pH to decline. These conditions are created when cattle are suddenly switched from a slowly fermentable carbohydrate(fiber) to a rapidly fermentable carbohydrate (starch).
Additionally, the primary acid produced from the fermentation of fiber is called acetic acid or acetate. Acetate is a weak acid and allows the ruminal pH to remain more neutral (pH of 6-7); whereas propionate or propionic acid, which is produced during the fermentation of starch, is a stronger acid that reduces pH more rapidly. As cattle are shifted from forage to concentrate as the primary energy source in the diet, the bacterial population shifts to one that favors rapid fermentation and propionate production and subsequently reduces ruminal pH to a range between 5 and 6, dependent upon the roughage to concentrate ratio. A pH of 5.6-6.0 poses no real risk to the animal’s health and is common in rapidly gaining cattle fed concentrates; however, if pH continues to fall below 5.5, the subsequent environment is conducive to the growth of bacteria, which produce lactic acid as a byproduct. For this reason, animals with a ruminal pH of 5.2-5.6 are considered to have subacute acidosis. Lactic acid is a strong acid and is not one that cattle can use efficiently for productive ends. It is in this acidic (pH < 5.2) environment that symptoms of acute acidosis are likely to be observed. These symptoms will typically present as cyclical oscillations in eating behavior. Calves with a functional rumen that have been improperly adapted to concentrate will often binge. Binge eating overloads the rumen with highly fermentable carbohydrate and acts as a primer for the acidic condition. As the pH of the rumen drops, the animal feels ill and goes off feed. When pH rises again the calf feels better, and because of hunger induced by an extended time without feed, then overeats again and the cycle repeats. If this cycle progresses, it can result in higher death loss in the herd. Death is usually the result of secondary infections caused by chronically low ruminal pH.
Animals do not need to experience acute acidosis to lose productivity. It is commonly thought that subacute acidosis is responsible for greater losses in production than acute-phase acidosis because the disease is harder to detect and therefore not treated.
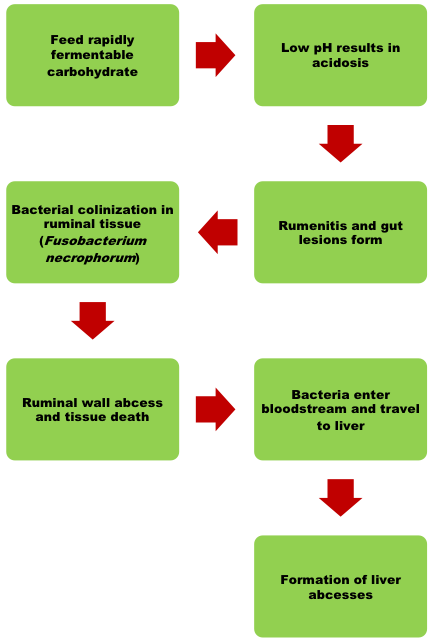
These infections begin in the rumen due to acid corrosion of the rumen wall. The sores that form will become infected and provide a route of egress for ruminal bacteria into the bloodstream. In some instances, the condition can affect normal keratogenesis, causing increased pressure in the hoof and thickening of the hoof wall. An example of this condition is shown in Figure 3. This condition is often manifested by long toes, and in cases where bacterial endotoxins are released, can result in a condition known as founder or laminitis.

The blood that leaves the digestive viscera flows directly to the liver so that absorbed nutrients can be processed and distributed to body tissues. When bacteria are introduced to the bloodstream and allowed to establish in the liver, then the hepatic tissue also becomes affected and the formation of liver abscesses begin (Figure 4). Severe liver abscess will result in reduced feed intake, gain,feed efficiency, and carcass yield. This condition is one of the challenges most commonly faced by the fed cattle industry because livers that show signs of current or previous infection cannot enter the human food chain. Liver abscesses are the leading cause of liver condemnations from carcasses in today’s packing plants, accounting for almost one-third of livers that come through the plant. Livers are currently worth about three dollars to the packer, and while this doesn’t represent a massive financial loss, the true cost of liver abscesses are more accurately measured in the feedlot where the effect of liver abscess is much costlier. Studies have shown that liver abscesses are responsible for reductions in feed intake and efficiency, gain, finished weight, subcutaneous fat thickness, and can account for a up to a 21-fold increase in carcass trim (Maday, 2018). This means that prevention must be a priority.
Prevention:
As discussed in the previous section, acidosis is often the result of improper acclimation to a high concentrate diet. The problem may be avoided by the formulation of a series of transition diets and a longer warm-up period to full feed. Transition diets should contain 40-60% roughage to start and the formulation should be slowly modified over three to four weeks to include less and less roughage. Roughage contained in high concentrate diets should not be ground too finely. A relatively large particle size is necessary to supply what nutritionists refer to as effective Neutral Detergent Fiber (eNDF). More commonly known as “scratch factor,” eNDF is the component of fiber that provides physical stimulation to the ruminal lining, which promotes ruminal contractions and rumination. The textbook definition of eNDF is “any fiber particle that will not pass through a1-millimeter screen (Van Soest, 1994). In reality the larger, more coarse particles make up the bulk of the solid phase or “raft” of the rumen contents. The more developed the raft, the slower the rate of passage through the rumen becomes, and the higher the digestibility of all components including those fibers less than 1 millimeter (Van Soest, 1994). Therefore, in Southeastern cow-calf systems it is advisable to ensure that at least 40% of dietary dry matter is composed of large particle (≥1 inch) fiber. Even in high concentrate diets, 8-10% roughage should be maintained as a minimum. Roughage in the diet stimulates cud-chewing or rumination. This behavior allows saliva to continuously be reintroduced to the rumen contents. Saliva contains bicarbonate salts and other buffering agents that help to maintain pH. Allowing adequate bunk space also helps to reduce competitive overconsumption. For high-risk calves a minimum of 18-24 inches per head should be adequate. In older, fat cattle, 10-12 inches should be acceptable. Sources of roughage may include stored forages (hay, silage, and baleage), cottonseed hulls, rice hulls, gin trash, peanut hulls, and crop residues (cornstalks or soybean stubble). Ionophores such as Rumensin® have been shown to reduce the incidence of acidosis when included in concentrate diets using label- recommended dosage.
White Muscle Disease
White muscle disease (WMD), also known as “nutritional muscular dystrophy,” causes degeneration of the skeletal and cardiac muscle of rapidly growing calves. This disease is most commonly the result of a selenium and/or vitamin E deficiency; although these deficiencies may also be present much earlier than the onset of clinical WMD with symptoms like decreased fertility and immune function. There are two presentations possible with WMD: cardiac and skeletal. Very little can be done to treat or alleviate the WMD in the cardiac muscle because onset is extremely rapid and quickly results in death. The skeletal type of WMD is more commonly observed and diagnosed ante mortem. Calves with skeletal WMD will present with stiffness and muscle weakness.
In the early stages of WMD, the animal’s appetite often remains intact; however, the inability to nurse or the regurgitation of milk through the nose has been seen in young calves due to muscle fatigue in the neck and upper airway. As the disease progresses, calves will lose the ability to stand for extended periods of time and may exhibit respiratory symptoms as the diaphragm and intercostal muscles become affected. Postmortem examination of affected animals often reveals discolored muscles that have a dry texture. Fibrosis and calcium deposition in the affected muscle present with a white streaking that gives WMD its name.
Prevention:
As with most nutritional disorders, dietary management and appropriate supplementation are often the keys to prevention. Selenium and vitamin E can be provided through a trace mineral supplement. Typically, minerals are formulated for 4 ounces/head/day intake. At this level of intake, mineral supplements should contain 26 parts per million (ppm) of selenium. Most cases of WMD in the Southeast are attributable to selenium deficiency.Vitamin E contribution to the prevention of WMD is more difficult to explain, as actual dietary recommendations for cattle at different stages of production have yet to be identified; however, it is known that vitamin E and selenium have a strong interaction with each other in the body.
Treatment:
Skeletal WMD can be alleviated with an intramuscular injection of vitamin E and selenium. If WMD is suspected, contact a veterinarian. Usually, 1 milligram of selenium and 68 international units (IU) of vitamin E per 40 pounds of body weight is considered a suitable dose. Supplemental selenium and vitamin E should be provided alongside treatment to correct the environmental conditions that led to deficiency.
URINARY CALCULI
Urinary calculi are mineral deposits or stones that accumulate in the urinary tract of cattle and small ruminants. These deposits often are rough and abrasive and can cause irritation to the urinary tract and persistent bladder infection which is a telltale sign of urinary calculi. In highly severe cases, these stones may completely block the flow of urine through the tract. This is particularly prevalent in male animals. If the blockage is not relieved, the urethra or bladder may rupture. A rupture of this nature may flood the abdominal cavity with urine. This urine can then be reabsorbed into the bloodstream causing toxemia and death within 48 hours. Initial signs of urinary calculi are typically urine leakage and straining to urinate. Other symptoms are associated with abdominal pain (e.g., foot stomping, kicking at the belly, and infrequent urination).
Prevention:
Urinary calculi are usually either phosphorus- or silica-based. Phosphorus is the more common of the two types and may be associated with any nutritional program where excess phosphorus is fed. Prevention may be achieved through dietary control of phosphorus intake, acidification of urine,and increasing urine volume. Also, it is important to focus on maintaining a calcium: phosphorus ratio of at least 1.5:1. It is important to note that a calcium: phosphorus ration up to 6:1 will not cause negative effects. For more information on mineral nutrition in beef cattle see UGA Cooperative Extension Bulletin 895 (Stewart, 2007). This will regulate the uptake of phosphorus so that concentrations stay within an acceptable range. Ensuring that adequate water is supplied to the animal is of paramount importance for the prevention of urinary calculi. Additionally, ammonium chloride may be fed. Ammonium chloride will acidify the urine and help to dissolve any stones that may form assuming they are not too large. The recommended feeding level of ammonium chloride is 1 to 1.5 ounces/head/day. Expect urine output to increase moderately as a result of feeding ammonium chloride salt.
Treatment:
The treatment of urinary calculi using ammonium chloride has been covered in the previous section. In reality, treatments that are designed to dissolve stones have only enjoyed limited success. In clinical cases, surgery is probably the most effective treatment, but as with most surgeries, the cost is relatively high and should be considered against the value of the animal, especially in the case of intact bulls who will no longer be able to be used as breeding animals. This is a decision that has to be made quickly, because if urine flow becomes completely blocked and the bladder does rupture, the animal loses any effective salvage value as the carcass will be contaminated with elevated levels of urea and must be condemned.
Polioencephalomalacia
In order to understand the effects of polioencephalomalacia (polio), it is necessary to understand thiamine. Thiamine, also known as vitamin B1, is required primarily by the body’s central nervous system to function normally. Polio disrupts the metabolism of thiamine in the body, causing mild to pronounced neurological deficits. Polio is most common in rapidly growing cattle fed a high-concentrate diet because this class of animals are more likely to encounter risk factors that lead to decreased thiamine activity. Most commonly, decreased thiamine concentration in the diet or high levels of dietary sulfates are the cause of polio in growing and feedlot cattle. Additionally, increased thiaminase (the enzyme that degrades thiamine) activity in the rumen can be a cause of polio in grazing cattle if they are given access to bracken fern (Pteridium aquilinum), a toxic plant that contains thiaminase.
Thiamine deficiency in the diet is almost exclusively found in high-concentrate, low-roughage ration fed for increased gain in feedlots or accelerated growing programs. Increased starch content from cereal grains causes a reduction in ruminal pH, as discussed in the section on rumen acidosis. Another side effect of this decrease in pH is the subsequent increase in the number of thiaminase-producing rumen bacteria which degrade dietary thiamine before it can be absorbed.
Sulfur, if fed in excess, can cause polio due to their interference with thiamine absorption. It is common to find high levels of sulfates in molasses as well as corn coproducts such as corn gluten feed and distillers grains. If these products are fed at a high inclusion rate in the overall diet without testing to determine sulfur concentration, it is possible to cause a polio outbreak. It is recommended that sulfur should not exceed 0.4% of the total diet.
Symptoms of polio are consistent with other neurological conditions and may include blindness, head pressing, uncoordinated movement, “marching” or “goose stepping,” lateral recumbency (lying on side with legs and head outstretched), muscle spasms, convulsions with paddling movement, and eventual death. Onset is typically sudden and can be startling. Infection and other issues may be ruled out by normal body temperature and rumination patterns.
Prevention:
All risk of polio can be controlled at the dietary level. For Southeastern producers, this may mean simply avoiding high-concentrate or high-sulfur diets when possible. If these types of diets are a necessary component of the management system, it is important to obtain a nutrient analysis to assess the concentration of sulfur. Also, make an effort to maintain neutral rumen pH by following the formulation guidelines discussed in the section on ruminal acidosis complex. Thiamine is sometimes supplemented in the diet or as a mineral supplement at a rate of 3-10 ppm, but this may not always be economically feasible and will likely not help avoid polio, because the primary causative mechanisms degrade thiamine in the rumen and interfere with absorption. Specifically, dietary supplementation of thiamine will not be a successful method to avoid polio. This is because the reason for the thiamine deficiency is that sulfur blocks proper absorption, so adding additional thiamine through the diet so it has to be absorbed through the gastrointestinal tract will not work due to the absorption-blocking properties of sulfur. The circulating levels of thiamine in the blood must be raised, but this must be accomplished by circumventing the rumen and associated absorptive mechanisms which have been affected by the sulfur- or thiamine-degrading enzymes in the diet.
Treatment:
Contrary to what may be inferred from the previous section, polio is actually easy to treat. The key is timing. Start by removing animals from any high-sulfur supplements and check pastures for toxic weeds such as bracken fern. Polio has to be treated as quickly as possible after symptoms present. Thiamine should be administered at a rate of 5-7 milligrams per pound intravenously. This should be followed by 5-7 milligrams per pound as intramuscular injections twice daily with the same dosage for the next two to three days. If cattle are not treated early in the disease’s progression, neurological systems may become permanent, although the severity may be reduced.
Prussic Acid Toxicosis
Although not technically a nutritional or metabolic disorder, the prevalence of prussic acid toxicities in Georgia is common enough to be considered an annual problem. Prussic acid, also known as hydrogen cyanide (HCN) was discovered in certain members of the sorghum family in the early 1900s. Since that time, HCN production has been found in two prevalent plant families in Georgia: the sorghum family, including forage and grain sorghum, Sudan grass, and Johnsongrass, as well as the Prunus family, including black cherry, chokecherry, pin cherry, and Carolina laurelcherry (Ball et al., 2015; Wahlberg, 2007).
Within the plant, HCN is simply a component of a larger molecule known as dhurrin (Vough, 1978). Dhurrin is known as a cyanogenic glycoside and is completely harmless in its natural state; however, when plants are stressed, a two-step process is triggered that results in the release of HCN from dhurrin (Vough, 1978). So why does the plant have to be stressed for this to happen? Because two essential components (i.e,. enzymes) of the reactions which release HCN are compartmentally separated from dhurrin in the plant cell. This means that something must happen to disrupt the integrity of the cellular structures that separate these ingredients. The most common stressor associated with prussic acid toxicosis is freeze or frost damage (Ball et al., 2015). As the cell freezes, expansion can cause membranes and cellular structures to be ruptured, but freeze damage is not the only mechanism by which HCN-producing plants can become toxic. Drought, wilt, herbicide damage, heavy nitrogen fertilization, plant disease, and physical damage can also cause the release of HCN into plant tissues.
If an animal consumes HCN-contaminated plant material, the compound will interfere with normal respiration at the level of the cell. This means the animal is still able to breath and even transport oxygen through the blood, but the cell cannot accept the oxygen from the blood because the HCN is already blocking the binding site. The first symptom of HCN toxicity is hypoxia or oxygen starvation.
If a large amount of contaminated material is consumed, the animal will experience muscle tremors and death, likely within minutes (Ball et al., 2015). If smaller amounts are consumed over an extended period, the process slows. The animal will begin to salivate heavily and experience a rapid breathing rate and muscle tremors. Eventually the damage will become so extensive that the animal will develop staggers and collapse. Contrary to nitrate toxicity where the blood will appear chocolate brown, during HCN toxicity, the animal’s mucous membranes will be bright red because the blood is fully oxygenated.
Prevention:
As mentioned earlier, there is no preventing acute toxicosis if contaminated material is ingested in large amounts. A veterinarian should be contacted immediately if any of the aforementioned symptoms are observed.
From a management perspective, producers have more control. Fence lines and grazing areas should be cleared of any of the aforementioned cherry varieties. Cattle death from HCN in Georgia is more often associated with wilted cherry leaves than with HCN-producing forages. This is because producers who choose to utilize grain sorghums or Sudan grasses have been vigilant about grazing practices, whereas exposure to wilted wild cherry is often the result of an unforeseen circumstance. Summer annuals are an excellent option for improving forage quality, but there are some management concerns that should be well-thought-out if grasses in the Sorghum family are under consideration for your program. Dhurrin, the HCN-containing component of these plants, is necessary for the growth of the plant; therefore, young growing plants as well as the topmost leaves of older plants contain the highest concentration of HCN. Similarly, elevated nitrogen rates, regardless of phosphorus, will increase the amount of HCN in the plant; however, unbalanced nitrogen and phosphorus can also be a contributing factor. Allowing plants to gain at least 18 inches in height before cattle are allowed to graze will give time to ensure that the soil chemistry is correct and that the plants are mature enough to ensure less volatile levels of HCN.
It is also important to remember that plant stress is the issue that will cause animal loss due to HCN from sorghums. So, allow at least a week (two weeks is better) or more to pass after a frost or, in the case of drought, to turn cattle onto these plants following a rain. Also, make sure that cattle are not hungry when turning out. In these circumstances, overconsumption can increase mortality more rapidly.
Treatment:
The treatment of prussic acid poisoning is rarely pursued because of the cost and the swiftness of action HCN has within the animal; however, treatment does exist and must be overseen by a veterinarian. This means that timing is vitally important in contacting a veterinarian if animals have been exposed to a source of HCN.
Nitrate Toxicosis
Again, although not technically a nutritional or metabolic disorder, the prevalence of nitrate toxicities in Georgia is common enough to be considered a yearly issue. An in-depth discussion of nitrate toxicosis is discussed in UGA Extension Circular 915 (Hancock, 2007), but we will provide the essentials here. Nitrates are a natural and healthy component of most forages. Nitrates are absorbed from the soil and metabolized by the plant to make necessary components such as amino acids and protein. The problem arises when the plant encounters stressful growing conditions such as drought or when the plant has had excessive amounts of nitrogen added in a given application. In these cases, the plant cannot metabolize nitrates quickly enough, and these compounds accumulate in the plant tissue, specifically in the lower stem.
When nitrates are introduced to the digestive tract of ruminants and other grazing animals such as horses, they are converted to nitrites. Under normal conditions, this is not a problem, as the animal’s metabolism reduces nitrite to ammonia and either uses it to form its own amino acids and proteins or further converts the compound to urea and excretes it as urine and feces. When nitrates in the forage reach a toxic level, the animal’s system will carry out the conversion to nitrite, but it cannot convert nitrite to ammonia quickly enough to keep pace with the increased concentration from the forage. In these cases, nitrite can be absorbed into the animal’s bloodstream. When this happens, an interesting, albeit dangerous, reaction can occur. Nitrite will bond to hemoglobin, the protein in blood that transports oxygen, and displace the oxygen, thereby depriving the tissues of oxygen. The severity of symptoms depends on the amount of nitrate consumed. Symptoms may include low milk production, abortions, and decreased fertility in mild cases, and tremors, labored breathing, rapid heart rate, and death in more extreme cases. In many of the reported cases in Georgia, animals are simply found dead due to an acute onset, usually after being turned in to a new pasture that is drought-stressed, or in winter, fed a hay or silage that was stored with excessive nitrate levels.
Prevention:
Due to the often rapid onset of nitrate toxicity, prevention is by far the best treatment. One of the most useful production practices for avoiding high- nitrate concentrations in forages is to split nitrogen fertilizer application throughout the growing season instead of administering the full rate at the beginning of the growing season. This limits the available nitrogen in soil without negatively impacting production. Testing forages is always recommended, but it is especially important when elevated nitrate concentrations are suspected. Producers should take several representative samples for analysis, because environmental conditions are often such that dangerous concentrations may be confined to certain areas of a pasture or hay field. Most county Extension offices are equipped with field kits to test for high nitrate concentrations and your local county Extension agent can help with testing. If it is determined that pastures contain hot spots with elevated nitrates, cattle should be limit-grazed or removed for 10-14 days after a drought-ending rain to allow time for nitrate levels to normalize. Hay should not be harvested for a similar amount of time. Ensiling forages has been shown to reduce nitrate levels from 30-60% (Hancock, 2007), but it is important to store properly and allow ample time for fermentation to take place before feeding. If forages are stored with high nitrate levels, it is imperative to test the forages prior to feeding, as concentrations can change. Once the analysis has been done, consult your county agent for help formulating a ration that adequately dilutes nitrates to an acceptable level for your cattle.
Treatment:
Prevention is the primary method of control for nitrate toxicosis due to the often rapid onset of symptoms; however, if nitrate toxicity is diagnosed, a veterinarian can administer an intravenous infusion of methylene blue and saline which will restore the oxygen-carrying capacity of the blood. This course of treatment is impractical in most cases because pharmaceutical-grade methylene blue is difficult to find and carries a withdrawal of 180 days.
SUMMARY
In the previous pages, we have highlighted many of the common ailments of Southeastern beef cattle that are attributable to nutrition. This is by no means a complete list and has been compiled more as a collection of most-likely suspects. Generally, nutritional disorders are easily corrected, but not always easily detected. For more information on various nutritional or metabolic disorders in beef cattle, contact your local county Extension agent by calling 1-800-ASK-UGA-1.
References:
Ball, D. M., Hoveland, C. S., & Lacefield, G. D. (2015). Southern forages (5th ed.). International Plant Nutrition Institute.
Gadberry, S., & Powell, J. (2011). Nutritional disorders in beef cattle (Publication No. FSA3071). University of Arkansas Cooperative Extension. https://www.uaex.uada.edu/publications/PDF/FSA-3071.pdf
Hancock, D. W. (2007). Nitrate toxicity (Publication No. C915). University of Georgia Cooperative Extension. https://extension.uga.edu/publications/detail.html?number=C915
Maday, J. (2018). Liver abscesses: Beyond just liver condemnation. Bovine Veterinarian.
Stewart, L. (2007). Mineral supplements for beef cattle (Publication No. B895). University of Georgia Cooperative Extension. https://extension.uga.edu/publications/detail.html?number=B895
Van Soest, P. J. (1994). Energy Balance. In Nutritional ecology of the ruminant (2nd ed., pp. 385–401). Cornell University Press. https://doi.org/10.7591/9781501732355-025
Vough, L. (1978). Preventing prussic acid poisoning of livestock (Circular No. 950). Oregon State University Cooperative Extension Service. https://forages.oregonstate.edu/fi/topics/pasturesandgrazing/grazingsystemdesign/preventingprussicacidpoisening
Wahlberg, M. L. (2007, August). Nitrate and prussic acid toxicity risk to cattle health. Virginia Cooperative Extension Service Livestock Update. https://www.sites.ext.vt.edu/newsletter-archive/livestock/aps-07_08/aps-804.html
Status and Revision History
Published on Feb 14, 2019
Published with Full Review on Jul 21, 2022


























































