Introduction
In recent years, global climate change has been one of the most frequently discussed scientific ideas in the popular press. Terms like “global warming” and “greenhouse gas” have been politicized with little discussion of what they really mean, shedding more heat than light on scientific discussions of changing climate. Concerns about the relative contributions of greenhouse gases from different industries have caused much finger pointing without making it clear how the gases are produced and what options are available for management of the gas emissions. In particular, agriculture has been identified as one of the major contributors to greenhouse gas emissions, and this has caused consumers to question the production practices used in modern agricultural systems. Specific focus has been placed on cattle production because these animals digest feed by a process called “enteric fermentation,” which produces methane as a by-product. The purpose of this bulletin is to explain the basic debate surrounding methane production from livestock, in particular how it is produced in the rumen.
Additionally, cattlemen are used to dealing with risk management and need to take changes in climate into account when planning strategies for farm management and marketing in the future. In the beef cattle industry, success is based on consumers’ willingness to purchase our product, and the modern consumer is inundated by the media’s perception that traditional beef production is bad for the environment. Therefore, it is important to understand the sources and potency of the common greenhouse gases in order to develop strategies for their reduction in the modern beef cattle industry.
Understanding the Greenhouse Effect
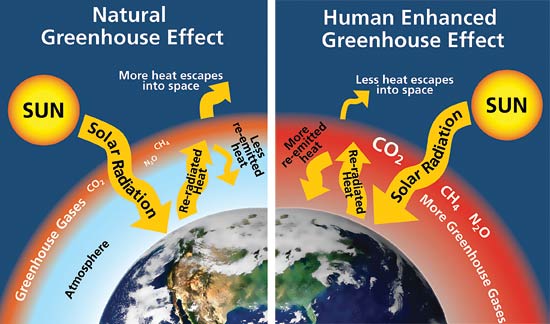
It is important to understand what is meant by the terms “greenhouse effect” and “greenhouse gas,” as well as how these gas emissions are measured. The greenhouse effect is a term that has been associated with the modern environmental movement beginning in the 1980s and 1990s; however, the theory was first hypothesized by Joseph Fourier in 1824 and fully quantified by Svante Arrheniius in 1896 (Held & Soden, 2000). The basic idea is that light from the sun passes through Earth’s atmosphere and is absorbed by the Earth’s surface. This warming of the surface emits heat energy, which moves back into the atmosphere as infrared radiation. Some of this radiation passes through the atmosphere and is released back into space. Greenhouse gases absorb and re-emit this radiation back toward the Earth, which increases surface temperatures. The natural greenhouse effect is an important part of Earth’s energy balance, keeping the surface temperature in a range that is needed to support life. Calling it “greenhouse effect” is really a misnomer, since real greenhouses work by using solid glass barriers to trap warm air inside enclosures rather than trapping infrared light.
In recent years, concentration of greenhouse gases has been increasing in the atmosphere. Climatologists have become concerned that too much heat is being trapped in the Earth’s atmosphere by these gases; thus, raising temperatures at the surface to levels that are believed to stress people and animals, as well as negatively affect vegetation and the water balance. The increase in gases have come from a variety of sources, including burning of fossil fuels, changes in land use (such as tropical deforestation and draining of wetlands), and both agricultural and commercial production. Stories about greenhouse warming in the popular press often present images of melting ice caps, stranded polar bears, and reduced air quality followed by a montage of scenes of modern agricultural practices, implying that agricultural production is solely to blame for the increasing temperatures. These tactics are used by some advocacy groups to generate public sympathy for a specific cause rather than to improve scientific knowledge.
What Are Greenhouse Gases?
The term greenhouse gas refers predominantly to four compounds: 1) carbon dioxide (CO2); 2) methane (CH4); 3) nitrous oxide (N2O); or 4) fluorocarbons. Carbon dioxide is the most prevalent greenhouse gas by volume, and it is the standard by which the potency of other greenhouse gases is measured (Faulkner, 2010). Carbon dioxide enters the atmosphere through respiration, certain chemical reactions (for example: manufacturing of cement), and the combustion of coal, natural gas, oil, solid waste, trees, and wood products (EPA, 2011).
“Global warming potential” is the system used to standardize the potency of greenhouse gases based on carbon dioxide equivalency (EPA, 2011). For simplicity’s sake, we will define global warming potential as the amount of infrared light radiated by any greenhouse gas relative to carbon dioxide. Methane is a byproduct of the decay of organic material; thus, potential sources include swamps, bogs, landfills, and compost. Methane from livestock is classified as anthropogenic, meaning that it originates from a living organism, and is classified as either manure derived or enteric. Enteric methane is produced in the rumen, and approximately 98% is exhaled by passive expiration through the nose and mouth. Often we hear “cow farts” being blamed for methane production and subsequent climate change. This is a massive misconception as the vast majority of greenhouse gas production from cattle comes from the rumen and exits through the nose and mouth.
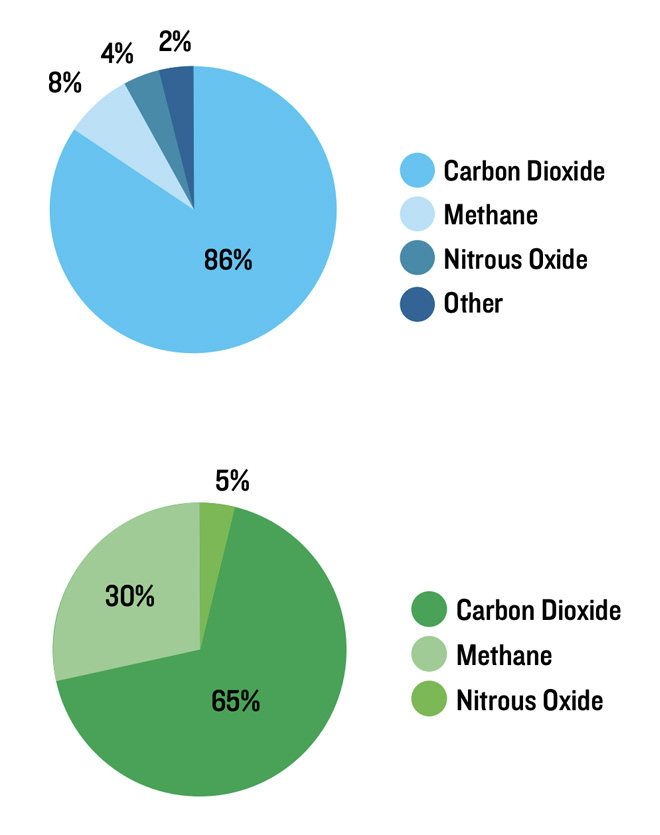
Nitrous oxide is emitted during the combustion of fossil fuels, solid waste, and certain industrial processes. The potency of methane and nitrous oxide is measured by CO2 equivalents. Carbon dioxide has a CO2 equivalence of one, while methane and nitrous oxide have a CO2 equivalence of 21 and 310, respectively, producing the increased global warming potential of these gases (Figure 2).
Although methane is responsible for only 30 percent of the greenhouse effect, it is the primary greenhouse gas associated with cattle production (Johnson & Johnson, 1995). While agriculture is only responsible for approximately 8% of total methane emissions, the livestock sector is responsible for almost one-third of agricultural methane emissions (EPA, 2011). This may seem like a small contribution to total greenhouse gas production, and it is, but today’s beef consumers are demonstrating a demand for a product that is produced with the least environmental impact possible; therefore, producers must understand and attempt to mitigate as much methane production as possible.
Methane production from cattle has been a point of interest for ruminant nutritionists for many years (Bratzler & Forbes, 1940; Johnson & Johnson, 1995). However, the focus of these studies was not the prevention or moderation of global climate change, but rather the energy inefficiency associated with methanogenesis (synthesis of methane) in cattle (Johnson & Johnson, 1995). From this we can see that although environmentalists and agriculturalists are often depicted as being on opposite sides of this argument, when it comes to reducing greenhouse gas production in cattle, there is common ground between these two groups.
Energy Source Affects Methane Emissions
Methane production in cattle begins with the first introduction of solid feed into the rumen (compartment of the digestive tract responsible for fermentation) at or around four weeks of age (Anderson et al., 1987). As the rumen develops, methane production continues to increase (Johnson & Johnson, 1995) and is estimated to plateau at 132 to 156 pounds annually for beef cattle or 240.3 to 277.78 pounds annually for dairy cattle (Johnson & Johnson, 1995). This data might suggest that the dairy industry would be the primary producer of enteric methane from cattle; however, the majority of dairy cattle consume a total mixed ration containing some high-quality forage and some grain. These highly digestible rations produce less methane per pound of feed than do simple forage-based diets. In contrast, the cow-calf sector has more animals consuming greater amounts of forage that is of variable quality. Due to these differences in population and diet quality, cow-calf operations in the beef industry are responsible for 58% of the methane emissions from cattle in the United States; the dairy industry accounts for 23% of emissions, while the stocker and feedlot operations account for 19% (EPA, 2011). Thus, increasing feed efficiency, diet quality, and total life cycle CH4 reduction, are important mitigation strategies.
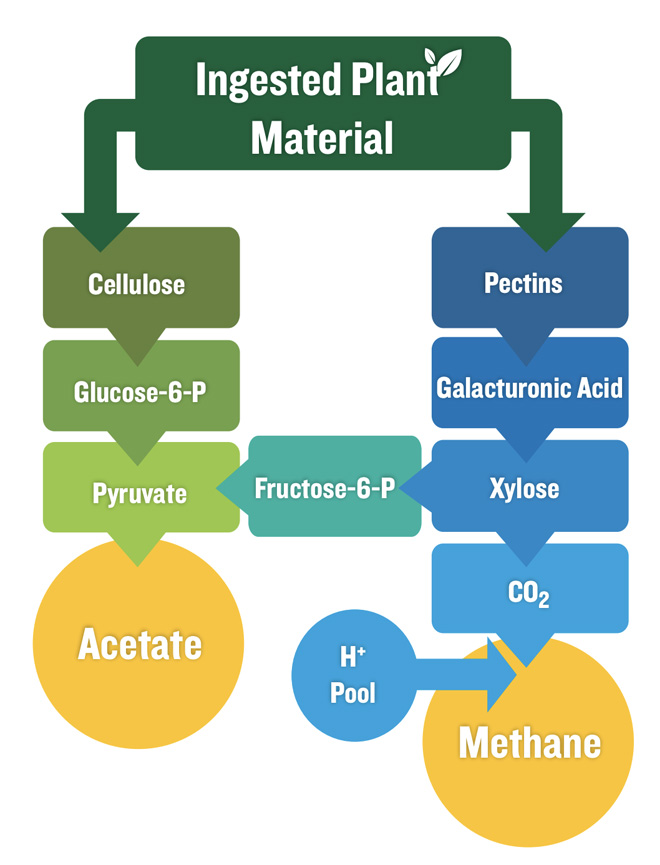
Measurements made by Johnson et al. (1993) indicate that methane production can account for energy losses of 2–12% of total energy intake by cattle. Carbohydrate source is one of the primary factors impacting rumen methane production. Carbohydrate fermentation by the microbial population in the rumen produces volatile fatty acids, which are used by the animal for energy. The two most prevalent volatile fatty acids are acetate and propionate. The fermentation of fiber produces increased proportions of acetate relative to propionate. The main product of fiber fermentation is acetate, whereas starch fermentation produces a larger fraction of propionate compared to fiber. During the fermentation process of fiber, soluble sugars are fermented to produce a sugar called xylose. Carbon dioxide is produced as a byproduct of this reaction, and it can be converted to methane through a series of biochemical reactions facilitated by methanogenic (methane-producing) bacteria (Russell & Gahr, 2000). The proportion of methane produced is dependent upon the amount of fiber in the diet compared to the amount of starch (Russell & Gahr, 2000).
Propionate can be produced from two distinct pathways. In forage-based diets, the “randomizing pathway” serves as the primary (90–95%) pathway for propionate synthesis, wherein carbon dioxide is produced and can be converted to methane (Figure 3). Conversely, in grain-fed ruminants the “acrylate pathway” produces 7090% of the propionate produced (Russell & Gahr, 2000). This is because the acrylate pathway is more robust in the acidic conditions created by rapid fermentation of starch, which results in rapid acid production, decreased rumination, and less buffering from saliva (Russell & Gahr, 2000). These acidic conditions are also less conducive to the growth of methanogenic bacterial populations in the rumen (Johnson & Johnson, 1995). Propionate production from the acrylate pathway is a “clean” process in terms of methane production because no carbon dioxide is formed, resulting in no opportunity for methane production.
For these reasons, concentrate diets result in less methane emitted per pound of meat produced compared with grazing systems (Clemens & Ahlgrimm, 2001). This is due to high starch diets combined with faster growth rates and shorter life spans in feedlot cattle (Clemens & Ahlgrimm, 2001). Beauchemin and McGinn (2005) monitored methane production from cattle fed 70% roughage (corn or barley silage) in the stocker phase and 80% concentrate (steam-rolled barley or dry rolled corn) in the feeding phase. Stocker cattle produced on average 0.38 pounds of methane per head per day when fed corn silage diets, which was greater than cattle fed barley silage—0.29 pounds per head per day (Beauchemin & McGinn, 2005). While methane emissions from cattle fed high starch diets from corn or barley during the feedlot phase were not different from each other, they did exhibit reduced methane emissions (0.14 and 0.18 pounds of methane per head per day for corn and barley, respectively) compared to the cattle that were stockered on forages (Beauchemin & McGinn, 2005).
Conclusion
Cattle are presumed to account for 73% of the 80 million tons of methane produced by livestock each year (Gibbs & Johnson, 1994). The fermentation of fiber is the primary reason for elevated methane emissions from cattle. Pasture-based or forage-finished operations require cattle to consume forages as their primary source of energy, and they require longer time on pasture to reach an acceptable fat thickness for harvest. Therefore, forage-based operations are likely to leave an equal if not larger carbon footprint compared to traditional feedlots.
References
Anderson, K. L., Nargaraja, T. G., Morrill, J. L., Avery, T. B., Galitzer, S. J., & Boyer, J. E. (1987). Ruminal microbial development in conventionally or early-weaned calves. Journal of Animal Science, 64(4), 12151226.
Beauchemin, K. A., & McGinn, S. M. (2005). Methane emissions from feedlot cattle fed barley or corn diets. Journal of Animal Science, 83(3), 653–661.
Bratzler, J. W., & Forbes, E. B. (1940). The estimation of methane production by cattle. The Journal of Nutrition, 19(6), 611–613.
Clemens, J., & Ahlgrimm, H.-J. (2001). Greenhouse gases from animal husbandry: Mitigation options. Nutrient Cycling in Agroecosystems, 60(1), 287–300.
Held, I. M., & Soden, B. J. (2000). Water vapor feedback and global warming. Annual Review of Energy and the Environment, 25, 441–475.
Faulkner, D. B. (2010). Potential to create carbon credits from beef production practices [Abstract]. Journal of Animal Science, 88(E-Suppl. 3), 59.
Gibbs, M., & Johnson, D. E. (1994). Methane emissions from the digestive processes of livestock. In M. J. Adler (Ed.), International anthropogenic methane emissions: Estimates for 1990 (Report No. EPA 230-R-93-010; pp. 2-1–2-44). Environmental Protection Agency.
Johnson, D. E., Hill, T. M., Ward, G. M., Johnson, K. A., Branine, M. E., Carmean, B. R., & Lodman, D. W. (1993). Ruminants and other animals. In M. A. K. Khalil (Ed.), Atmospheric methane: Sources, sinks, and role in global change (NATO ADI Series Vol. 113, pp. 199–229). Springer-Verlag.
Johnson, K. A., & Johnson, D. E. (1995). Methane emission from cattle. Journal of Animal Science, 73(8), 24832492.
Lallanilla, M. (2013). What is the greenhouse effect? LiveScience. https://www.livescience.com/37743-greenhouse-effect.html
Russell, R. W., & Gahr, S. A. (2000). Glucose availability and associated metabolism. In J. P. F. D’Mello (Ed.), Farm animal metabolism and nutrition (pp. 121–148). CABI Publishing.
U.S. Environmental Protection Agency. (2011). Inventory of U.S. greenhouse gas emissions and sinks: 1990–2009 (USEPA #430-R-11-005). http://epa.gov/climatechange/emissions/usinventoryreport.html
Status and Revision History
Published on Nov 07, 2015
Published with Full Review on Feb 27, 2019
Published with Full Review on Dec 01, 2022


























































