
- Canola Characteristics
- Canola Growth Requirements and Stages
- Canola Production Practices
- Fertilization
- Managing Weather Related Risks
- Canola Pest Management
- Harvesting and Handling Canola
- Canola Production Costs and Marketing
- Summary
Introduction
Canola is an agronomic crop grown in many countries for production of vegetable oil and meal. Demand for canola oil continues to increase worldwide. People consume canola oil and consider it to be a healthy oil with low levels of saturated fats. More recently, there also is intense interest in using canola oil to produce biodiesel to replace diesel fuel. Canola meal is normally fed to poultry and livestock as a source of protein.
Canola was grown commercially in Georgia, Alabama, South Carolina and northern Florida from the late 1980s through about 2000 with a peak production of about 25,000 acres. Commercial production stopped during the early 2000s with the loss of markets. Interest in production of canola in this region has been renewed by the development of on-farm crush facilities that now provide a local markets. Over the past several years acreage has steadily grown to more than 35,000 acres in the region.
Past experience shows that canola can be produced profitably in Georgia. In 1997, canola production averaged 27 bushels per acre, but many growers had fields that yielded more than 50 bushels per acre. Analysis at that time indicated that 30 bushels per acre was needed to cover production costs. With proper management, growers can expect yields of more than 40 bushels per acre.
An important consideration in producing canola is the ability to sell it at a convenient local market for a good price. Producers should find a buyer before committing to produce the crop and find out how canola will be priced.
Growing canola profitably takes planning and good management. All aspects of production from seed selection to harvest to marketing must be taken into account if the grower is to make a profit with this crop. Land preparation, fertility management, weed and other pest control, and timely harvest and marketing are all components of a good canola production package. Before you grow canola, dedicate yourself to make "best management practices" a part of your production system.
Canola Characteristics
Canola is a Brassica species and is closely related to turnips, collards, mustard and cabbage. Two species are grown worldwide as canola, Brassica napus and Brassica rapa. Only Brassica napus is grown in the Southeast. Historically Brassica napus was referred to as "rapeseed" (rape from rapus, which means turnip in Latin). Canola is a type of rapeseed that has been bred to have very low levels of erucic acid and glucosinolates. The term "canola" was coined by the Western Canadian Oilseed Crushers to specify a rapeseed that contains less than 2 percent erucic acid in the oil and less than 30 umol of glucosinolates per gram of the oil-free meal. Rapeseed not meeting these standards cannot be termed canola. In Europe, where the term "canola" is not preferred, canola quality rapeseed is often called "double-low" or "00" rapeseed.
Canola seed at maturity typically contains 38 to 42 percent oil. The oil has about 6 percent saturated fats, which is lower than any other major vegetable oil. Canola oil also has high levels of mono-unsaturated fat (oleic acid) and a favorable mix of mono- and polyunsaturated fats, which is desirable for human consumption.
Canola meal contains about 37 to 38 percent crude protein after oil extraction compared with 44 percent for soybean meal. The meal also contains about 11 percent crude fiber, which reduces its metabolizable energy as compared with soybean meal. Canola meal is used mainly as a protein ingredient in feed rations for livestock and poultry.
Conversely, rapeseed containing high levels of erucic acid (40 percent or more) in the oil is termed "industrial rapeseed" or HEAR, for high erucic acid rapeseed. Oil from HEAR varieties is used mainly as a lubricant for industrial purposes. Meal from HEAR varieties usually has high levels of glucosinolates, making it unsuitable as an animal feed. Currently HEAR rapeseed is not grown in Georgia.
Canola seed at maturity typically is black, although varieties with a yellow seed coat may be available in the future. It takes 110,000 to 140,000 seeds to make 1 pound of canola. Seeds measure 1/32 to 3/32 of an inch in diameter, but seed size varies with variety and environmental effects. One bushel of No. 1 grade canola weighs 50 pounds.
Canola Growth Requirements and Stages
Canola is a cool-season plant and is grown in Georgia as a fall-planted winter crop. Canola has six major growth stages: (1) seedling, (2) rosette, (3) bolting, (4) flowering, (5) pod-fill and (6) maturity. (See Figure 1.)
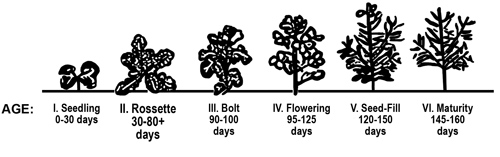 Figure 1. Canola growth stages.
Figure 1. Canola growth stages.The seedling stage occurs during the first 25 to 30 days in the life of the plant. During this time, three to five small leaves develop in addition to the seedling dicot leaves. The rosette stage occurs over the next 60 to 100 days, depending upon variety earliness and winter temperature. During this period, plants accumulate 12 to 20 leaves and appear much like turnips or leafy greens.
Bolting is when canola flower stalks begin to elongate. The flower stalk elongates rapidly and develops 6 to 12 nodes or "joints." Flower heads form soon after bolting begins. Plant height is usually in the range of 3 to 5 feet.
In winter-type canola, bolting does not occur until plants have received 700 to 800 hours of chilling temperature. In areas with very mild winters, bolting of winter-type canola may occur very late or not at all. Spring-type canola varieties require very little or no winter chill hours for vernalization and bolting. For this reason, winter-type varieties are best suited for northern Georgia (Plant Hardiness Zone 7), and spring-type canola varieties are best suited for the Georgia Coastal Plain area (Plant Hardiness Zones 8A and 8B). (See Figure 3)
Canola flowering usually begins around March 1 and continues for four to five weeks. The flower stalk growth is indeterminate and axillary flower stalks may form at lower nodes, especially if the canola population is sparse. Canola flowers are perfect, that is they contain both male and female structures. New flowers form daily. Self pollination occurs during pre-dawn hours. Bees aid in pollination but are not required for pollination (see section on bees). Typically, only about half of the canola flowers form seed pods. Flower abortion usually occurs at times of "hard" freezes (< 25 degrees F), at very high air temperatures and/or during nutrient or drought stress.
Pod and seed development begins soon after pollination. Canola pods are siliques that contain rows of seeds on each side of a central membrane called a "septum." Canola pods also have two outer hulls, each covering a row of seeds. Each canola pod typically contains 15 to 30 seeds. The seed-fill period usually occurs over a four- to six-week period from late March to early May.
Physiological maturity occurs when all pods have started turning brown and when the cotyledons within the seeds have turned from green to yellow. For early maturing canola varieties, physiological maturity will usually occur by early May. For late spring-type varieties and winter-type varieties, physiological maturity may not occur until late May or early June. Canola stalks dry from the top downward. Canola is ready for direct harvest when seed have dried to 9 percent moisture. At this time, the lower canola stalk may still be quite green.
The outer seed pod walls form a complete abscission layer at maturity and remain only loosely attached. As such, canola is highly susceptible to seed shattering with weathering and plant disturbance.
Canola Production Practices
With good varieties, fertility and cultural management, canola yields of 40 to 50 bushels per acre are realistic. Careful attention to variety selection and management is necessary to make this possible. Use the following guidelines.
Select Adapted Canola Varieties. Generally, wintertype varieties are best suited for northern Georgia, and spring-type varieties are best suited for southern Georgia. Only a very limited number of adapted varieties are commercially available. If possible, plant varieties with resistance to blackleg disease. Susceptible varieties can be completely destroyed by blackleg. This practice is critical if planting on land where canola or Brassica vegetables have been grown in the past three years.
Planting both early and late maturing varieties helps ensure consistent canola yields. Early maturing canola varieties tend to have highest yields but are more vulnerable to winter freeze injury than late varieties. On the other hand, late-maturing canola varieties are more vulnerable to spring drought than early varieties. Planting early and late varieties spreads out these risks. In addition, planting early and late varieties spreads out the optimum time for harvest and the subsequent planting of a summer crop.
The University of Georgia Statewide Variety Testing Program evaluates canola varieties at a limited number of test locations. Check results of the most recent evaluations at www.griffin.uga.edu/swvt. Click on the canola link.
Select Proper Fields and Prepare Soil Correctly. Well-drained, productive row crop soils are generally well-suited for canola production. Good wheat and cotton land can produce high canola yields. Avoid planting canola on poorly drained soils or deep sands. Under prolonged wet conditions, canola typically becomes diseased, winter kills easily and yields poorly. On deep sands, nitrogen and sulfur deficiencies can be problems for canola production even with efforts to supply these nutrients. Good Georgia soils for canola include Cecil, Greenville, Orangeburg, Tifton and Dothan series.
Deep till all sandy textured soils to depths of 10 inches or more to disrupt hardpans. Deep chisel or bottom plow to accomplish this. Firm and level the soil surface after tillage to prepare for planting. This can usually be accomplished with a light drag at the time of deep tillage. Sometimes a light discing may also be needed. Deep rooting of canola is essential for good growth, winter survival and high yield potential.
Avoid re-compacting the soil after tillage. One tractor pass re-establishes 80 to 90 percent of soil compaction in the tire track area. Re-compaction of the soil can usually be prevented if deep tillage, soil leveling/firming and planting are done in the same trip. Set up and establish lanes for all subsequent field traffic.
Rotate canola with other crops. Rotate canola so that winter legumes, canola or other Brassicas are planted on land no more than one in three years or preferably every four years. This is necessary to help suppress soil-borne diseases like white mold (Sclerotinia) and blackleg. In addition, wild radish cannot be controlled in conventional canola. Careful attention to rotation and aggressive wild radish control measures in rotational crops can allow conventional canola to be grown in wild radish infested fields. Carryover residues from persistent broadleaf herbicides applied to cotton, peanuts and other crops can injure canola (see weed control section). Check past records to determine if canola can be grown safely in a given field.
Do not grow canola directly before or after other Brassica crops like cabbage, turnip, broccoli, etc. These Brassica species host many of the same diseases. Farms committed to the production of cabbage and leafy greens should NOT include canola in their rotations.
Studies and previous experience have shown that canola fits well in a rotation following corn, sorghum, tobacco, early-system soybeans and certain vegetables. Full-season soybeans, cotton and usually peanuts are harvested too late for timely planting of canola. Summer crops following canola include soybean, grain sorghum, millet and possibly late-planted corn. Canola is harvested too late for full-season cotton and peanuts. Doublecropping cotton after canola is feasible, but the delayed planting reduces cotton yield potential. Canola also tends to enhance cotton stand losses due mainly to increased severity of seedling diseases. Standard fungicide seed treatments and increased cotton seeding rate are recommended when planting cotton following canola. An additional in-furrow fungicide treatment also may be helpful.
Test the soil and apply needed fertilizers. Canola is unusually sensitive to low soil pH, especially at levels below 5.7. Apply and incorporate needed phosphorus and potassium ahead of planting. Canola requirements for nitrogen, sulfur, calcium, magnesium and boron are higher than required for some other crops. See the section on fertilization for specific instructions.
Apply a preplant incorporated herbicide. Chickweed and henbit can be controlled by using a preplant incorporated herbicide such as trifluralin. Post emergence herbicides can be used to control grassy weeds such as annual ryegrass. See weed control section for details.
Use proper planting rate and depth. Plant canola seed only 1/4 to 1/2 inch deep in a clean, firm seedbed. Canola stands and uniformity of emergence will be much improved if planting is made with a precision drill that has double disc openers, depth control devices and press wheels to firm soil around seed. A Brillion seeder or similar equipment suited for establishing small-seeded forages works well for establishing canola. Often the surface soil will be dry at the time of canola seeding. While planting in moist soil is preferred, planting in dry soil will usually gives satisfactory stands once rain occurs.
Plant only high quality canola seed that has been treated with a fungicide. A plant population of six to ten plants per square foot is optimal for high yields. Yield losses may occur when plant populations at harvest are less than three to four plants per square foot or more than fifteen plants per square foot.
If using a precision drill, plant about 4 pounds of canola seed per acre and plant in row widths of 6 to 12 inches. For drill without press wheels, use a light drag to firm soil around seed and increase canola seeding rate to 6 pounds per acre to help improve chances of getting a good stand.
Successful canola stands have been obtained with aerial seeding and by spreading with bulk blend fertilizer. This is not a recommended means of seeding canola but can work if the seeding rate is increased to 7-8 pounds per acre and if the soil is dragged lightly and irrigated after application.
Use optimal row spacing. Normally optimal row spacing is 6 to 8 inches. Canola yields well in wider rows, such as 12 to 15 inches, but wider rows may reduce the cropâ??s ability to compete with weeds. Row spacing of more than 15 inches is not recommended.
Plant at the proper date. Plant canola about one month before the time of the first expected killing frost. For north Georgia, this means that canola should be planted from mid-September to mid-October. For southern Georgia, canola should be planted from mid-October to mid-November. See the suggestions below and Figure 3 for the appropriate time to plant canola in your area.
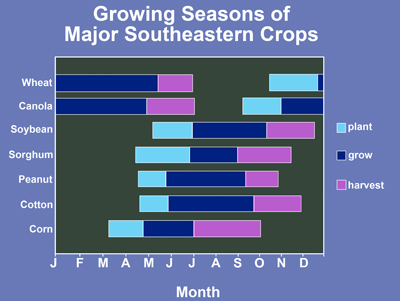 Figure 2. Planting, growing and harvest periods for canola and other major crops in the Southeast.
Figure 2. Planting, growing and harvest periods for canola and other major crops in the Southeast.
NORTH GEORGIA (Winter Types)
Plant Hardiness Zone 7A: September 10 to October 15
Plant Hardiness Zone 7B: September 15 to October 25
SOUTH GEORGIA (Spring Types)
Plant Hardiness Zone 8A: October 10 to November 10
Plant Hardiness Zone 8B: October 15 to November 15
Planting canola too early or too late increases risks of winter kill. Canola is most susceptible to winter kill just after emergence and at bolting. Late planting increases the odds of having small plants at the time of hard freezes. Conversely, very early fall plantings increase the odds that bolting will occur before late winter freezes have ended. Careful adherence to suggested varieties and planting dates for a given area will minimize chances of winter kill.
Fertilization
Nutrient Uptake
Well-drained productive soils and adequate fertilization are essential for obtaining top canola yields and seed quality. Past research in Georgia indicates that canola can take up more than 180 lb/acre of nitrogen (N) and potash (K) as well as significant amount of phosphorus, calcium, magnesium and sulfur (see Table 1). Fertilizer requirements are higher than those for soft red winter wheat production. Although canola takes up large amounts of nutrients from soil, not all of the nutrients are removed from the field at harvest.
Nearly 40 percent of the N taken up is removed with the seed, meaning a fairly high amount of N can be available to the summer crop following canola.
As in other oilseed crops, K is very important for moisture regulation, oil production and oil quality. Magnesium (Mg) and sulfur (S) are also important in the plant processes leading to oil production and can adversely affect yield and oil quality if deficient. Boron (B), a readily leachable essential micronutrient required for proper pollination, is especially important for canola. Deficiencies can cause suppressed growth, poor pod fill or pod abortion.
| Table 1. Total Nutrient Uptake by Canola. | ||||
| Grain Yield lb per acre (bushels per acre) | Average Nutrient Uptake (lb per acre) | |||
| N | P2O5 | K2O | S | |
| 2,000 (40) | 120 | 60 | 95 | 20 |
| 3,000 (60) | 180 | 90 | 145 | 45 |
| 4,000 (80) | 240 | 120 | 190 | 60 |
Liming and pH
Canola requires a soil pH of 5.8 to 6.5 for normal growth and yield (see Table 2). In Georgia soils, pH range should ensure adequate availability of calcium, magnesium and most micronutrients. If pH is too low, use dolomitic limestone to supply both Ca and Mg. Where pH is adequate and soil Mg levels are low, apply 25 lb elemental Mg/acre using a water soluble source such as potassium-magnesium-sulfate.
| Table 2. Effect of Soil pH on Canola Yield, Tifton, Ga., 1992. | |
| Soil pH | Grain Yield lb per acre, (bushels per acre) |
| 5.3 | 914 (18.3) |
| 5.6 | 1,933 (38.7) |
| 6.5 | 2,375 (47.5) |
| *Data from Gary J. Gascho, Dept. of Crop and Soil Sciences, Coastal Plain Experiment Station, University of Georgia, Tifton, Ga. | |
Nitrogen Management
Based on studies in Georgia, canola yields continue to increase at N rates up to 180 lb/acre for both spring and winter types. Recommended N rates for Georgia conditions vary from 135 to 180 lb N/acre. A nitrogen credit of 20 lb/acre can be given for canola following a legume such as peanuts or soybeans. Split applications are recommended by applying 50 lb N/acre at planting, 30 lb N/acre in mid-December, and 90 to 100 lb N/acre in mid-February before bolting. (See Table 3.) Ammonium sulphate can be used as the N source for at least one application to supply adequate S. Urea is a viable N source because volatilization should be low due to the low seasonal temperatures at application. Fertilizer N in excess of 10 lb/acre applied as starter, in direct contact with seed, will reduce germination.
| Table 3. Suggested Nitrogen and Sulfur Fertilization Regimes for Producing 45 Bushels per Acre. | ||
| Time of Application | Amount (lb) per Application | |
| Nitrogen | Sulfur | |
| At planting | 50 | 20 |
| 40-45 days after planting* | 30 | 0 |
| 80-95 days after planting* (about mid-February or prior to bolting) |
90 | 20 |
| * This application may be excluded if canola has normal green leaf color and growth. | ||
High N rates early in the season can cause excessive vegetative growth and even early flower formation, resulting in a greater chance of winter-kill. Split N applications will minimize these effects and reduce leaching losses. Later in the season, excessive N results in lodging, delayed maturity and reduced quality.
Boron and Sulfur
Canola has a relatively high requirement for both boron (B) and sulfur (S). In addition, both B and S are mobile in soil and can leach out of the root zone with excessive rainfall. Low organic matter soils common to the Southeast also contain little B or S in organic forms to be mineralized for plant uptake. Apply 1.0 lb/acre of B and at least 30 lb/acre of S, especially on sandy soils with less than 1 percent organic matter. Since B and S deficiencies can occur in fall or spring (depending on soil temperatures and rainfall), include both of these elements in preplant and spring fertilization practices if possible. Using ammonium sulfate as an N source can supply S in fall or spring. Also, B can be included in preplant fertilizer and as a foliar feed with insecticide or fungicide sprays in the spring. Foliar B should be applied at the rate of 0.25 to 0.5 lb B/acre. Foliar application of less than 0.25 lb B/acre is not economical. Demand for both B and S is greatest during flowering and pod fill, so supply these nutrients in spring prior to bolting.
Poultry Litter
Poultry litter (manure mixed with bedding) contains significant amounts of essential plant nutrients, making it a valuable fertilizer material for meeting the high fertility requirements of canola. The nutrient content of broiler litter can vary significantly, so we recommend that litter be tested for nutrient content by a reputable laboratory before determining application rates and value. Broiler litter normally contains about 3 percent N, 3 percent P2O5 and 2 percent K2O, making it a 3-3-2 fertilizer. This means 1 ton of litter normally contains about 60 lb of N, 60 lb of P2O5 and 40 lb of K2O. Based on current (spring 2007) fertilizer prices for N, P and K, this would give poultry litter a value of approximately $35/ton based as a fertilizer. Poultry litter also contains significant quantities of secondary and micronutrients (except for boron) and can help maintain proper soil pH and organic matter content, which can add to its value.
Recent research conducted on Coastal Plain soils in Georgia indicate that 4 tons/acre of broiler litter can meet the fertility requirements of canola. This rate, however, normally supplies excess phosphorous, which can cause potential surface water pollution. In addition, nitrogen availability, especially in early spring, is much less predictable compared to commercial fertilizer. Therefore, use poultry litter preplant, at a rate of 2 tons/acre followed by normal commercial fertilizer rates of N, S and B in the spring. This strategy should be best for meeting the nutrient requirements of canola while at the same time minimizing potential environmental problems.
Managing Weather Related Risks
Canola performs well as a winter crop in areas such as the Southeast, where winters are typically mild. Canola is generally less winter hardy than wheat, however, and is vulnerable to cold injury during severe winters. The level of cold tolerance in canola greatly depends upon growth stage, level of acclimation or hardening and, to some extent, variety. Canola is most winter hardy during the rosette stage and when acclimated prior to exposure to extremely low temperatures. It is most sensitive to winter injury during the seedling and reproductive stages of development.
Risks of Early Season Injury
Canola has very little cold tolerance at the time of emergence, and young seedlings can be damaged or killed by temperatures below 25 degrees F. About two weeks of growth are required for canola to properly acclimate and withstand lower temperatures. Figure 4 illustrates that the probability of a damaging freeze event shortly after emergence increases rapidly after the end of the recommended planting period. The risk of early season cold injury can be greatly reduced by strict adherence to the recommended planting dates. See the section on production practices for planting date recommendations in your area.
Risks of Mid-Winter Injury
Canola is most cold tolerant during the rosette stage, which, if planted during the recommended planting dates, coincides with our harshest winter months. The level of cold tolerance at the rosette stage is highly dependent upon variety and temperatures one to two weeks before a freeze event. For example, if temperatures have steadily declined in the weeks prior to an extreme cold event, canola may survive temperatures as low as 5 degrees F. However, if warm temperatures have prevailed just prior to the extreme cold event, canola will be less cold tolerant and vulnerable to damage by temperatures of 20 degrees F or lower.
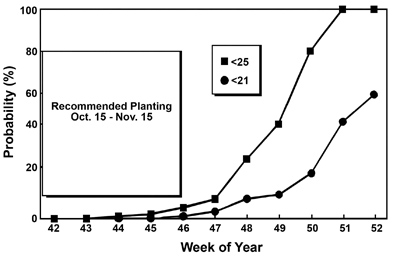 Figure 4. Average cumulative probability of minimum temperatures less than 21º or 25º F before a given week at Tifton, Ga. (The recommended planting time for canola in the lower Coastal Plain region is October 15 through November 15, which corresponds to week 42 and week 46, respectively.)
Figure 4. Average cumulative probability of minimum temperatures less than 21º or 25º F before a given week at Tifton, Ga. (The recommended planting time for canola in the lower Coastal Plain region is October 15 through November 15, which corresponds to week 42 and week 46, respectively.)Previous research shows that canola varieties vary in their ability to acclimate and, therefore, their ability to withstand low temperatures. Field injury ratings taken shortly after significant freeze events indicate large differences in cold tolerance among varieties. For example, Sponsor, a variety developed in Sweden, had little injury, while Oscar, an Australian variety, suffered severe damage. These results provide hope for the development of more cold tolerant varieties for our growing region and indicate that the risk of winter injury during the rosette stage could be reduced by selecting cold tolerant varieties when available.
Other factors such as stand density and nitrogen management also influence survival during a cold event. Risks of winter injury during the rosette stage can be reduced by achieving good uniform stands and managing nitrogen to promote early season growth while avoiding excessive or lush winter growth.
Risks of Injury during Flower
When canola enters the reproductive phase of development, it becomes vulnerable to cold injury during bolting and flowering. Flowering canola can be killed by temperatures of 20 degrees F or below. Risks associated with late spring freezes are best managed by selecting varieties that flower at the proper time for your production zone. Logically, earlier flowering varieties are at greater risk of cold injury than later flowering varieties. However, varieties that flower too late may experience yield reductions associated with high temperatures during flowering. Research indicates that varieties that flower during late February or early March are typically the highest yielding in plant hardiness Zone 8 (see Figure 3).
In summary, risks associated with winter injury are real but can be significantly reduced by strict adherence to recommended planting dates, establishing good stands early, managing nitrogen to promote early growth but to avoid lush winter growth, and by selecting varieties with appropriate flowering dates for your production zone.
Canola Pest Management
Canola responds well to an intensive scouting program. Periodic monitoring of insects, weeds, diseases and fertility can often reduce crop losses if problems are corrected in a timely manner. It is recommended that growers participate in a scouting program. Specific pesticide recommendations are updated annually and are available in the Georgia Pest Management Handbook, commercial edition at: http://www.ent.uga.edu/ pmh/Com_Canola.pdf
Weed Control
Problem Weeds in Canola
Italian (annual) ryegrass, Lolium multiflorum, is a problem weed in most crops that are grown during the winter months in Georgia. Italian ryegrass is a winter annual and reproduces by seed. Italian ryegrass has bright green leaves that are glossy on the underside and deeply ridged on the upper surface. Prominent auricles are highly visible at the junction of the leaf blade and sheath. The seedhead is a long slender spike with an alternate arrangement of spikelets. Trifluralin will provide only partial control of Italian ryegrass. Postemergence grass herbicides that can be used for ryegrass control in canola include Poast (sethoxydim), Select/ Arrow (clethodim), and Assure II/Targa (quizalofop). In 2010, a Poast-resistant population of Italian ryegrass was confirmed in Franklin County. However, this population was still susceptible to Select (clethodim).
Wild radish, Raphanus raphanistrum, is a common weed in small grain fields in middle and south Georgia. Wild radish has deeply lobed leaves attached by long petioles, stems with downwardly-pointing stiff hairs, and light yellow flowers. Light brown to reddishcolored seed are formed in a conspicuously ribbed seed pod that easily breaks into small segments. A related species, wild mustard (Sinapis arvensis), is often found in Georgia but may be distinguished from wild radish by the presence of sessile (lack a petiole) leaves in the upper portion of the plant and dark yellow flowers. Additionally, wild mustard forms black seed in a slender pod that does not break into individual segments. There is no herbicide available for use in conventional canola that will control either wild radish or wild mustard. Producers should not plant conventional canola in fields infested with these weeds. A better option would be to rotate infested fields to winter wheat for two to three years. Numerous herbicides are available that will control wild radish and wild mustard in winter wheat. Refer to the Clearfield canola section for information about wild mustard/radish control in herbicide-resistant varieties.
Other weeds such as chickweed (Cerastium vulgatum), henbit (Lamium amplexicaule), hairy vetch (Vicia villosa) and wild garlic (Allium vineale) can compete with seedling canola during establishment. Preemergence use of trifluralin is an important component for successful conventional canola establishment.
Conventional Canola
Trifluralin (Treflan, Trilin, and other brand names), clethodim (Select, Arrow, and other brand names), sethoxydim (Poast), and quizalofop (Assure II, Targa) are currently labeled for use in Georgia for weed control in conventional canola. Clopyralid (Stinger) also is registered for use in canola, but limited Georgia data is available for this herbicide. Since only a few herbicides are available for weed control in canola, producers must follow all crop production recommendations to successfully produce canola in Georgia. As with most crops, good weed control during the first six to eight weeks after emergence will be needed to prevent weeds from reducing yield. Canola is a relatively slow-growing crop after emergence. It is during this period of slow growth that weeds will be able to become established. Following the recommendations for seeding, fertilizing, seeding date and other cultural practices will help canola become established and increase its ability to compete with weeds. Canola generally will shade the row middles in six to eight weeks. After this period of time, canola is extremely competitive with weeds. In addition to the yield-decreasing effects of weeds, producers need to be aware that the presence of certain weed seeds, such as wild radish, wild mustard and wild garlic, may result in severe dockage and may decrease the marketability of canola seed. Due to similar seed size, it is extremely difficult to mechanically separate (clean) wild radish and wild mustard seeds from canola.
Herbicide-Resistant Canola
Clearfield Canola
Clearfield canola varieties are under development for production in Georgia. Consult your local county extension office for varieties adaptable to your area. All herbicides registered for use in conventional canola (trifluralin, sethoxydim, quizalofop, clethodim and clopyralid) also can be used in Clearfield canola.
Imazamox (Beyond) herbicide can only be applied to Clearfield canola varieties. Do not apply Beyond to conventional canola varieties or serious injury and/or death will occur. Beyond herbicide can be applied postemergence to Clearfield canola varieties after emergence but before blooming. Apply 4 oz/acre of Beyond 1AS with a nonionic surfactant (0.25% V/V) plus a nitrogen fertilizer (2.5% v/v of 28% or 32% N) to improve weed control.
Imazamox provides control of several grass and broadleaf species including wild radish. All weed species are best controlled when imazamox is applied to weeds that are less than 3 inches tall and actively growing. Clearfield canola has resistance to other imidazolinone herbicides.
Liberty-Link and Roundup Ready Canola
Canola varieties have been developed that are tolerant to postemergence applications of either Liberty (glufosinate) or Roundup (glyphosate). These herbicides are not registered for use on canola grown in Georgia at this time, but they may become available in the future.
Trifluralin Use in Canola
Preplant incorporated applications of trifluralin will control some winter annual grasses, henbit and common chickweed in canola (Table 4). Trifluralin will not control wild radish or wild mustard. Recommended rates of trifluralin for canola are shown in Table 5. Trifluralin must be incorporated within 24 hours of application to a depth of 2 to 3 inches. Incorporation equipment similar to that used in soybeans or cotton may be used.
| Table 4. Effectiveness of Trifluralin on Various Weeds. | |
| Weed Species | Controlled with Trifluralin |
| Annual bluegrass | yes |
| Italian (annual) ryegrass | partial control |
| Cheat | yes |
| Downy brome | yes |
| Volunteer wheat | partial control |
| Catchweed bedstraw | no |
| Common chickweed | yes |
| Curly dock | no |
| Cutleaf evening primrose | no |
| Field pennycress | no |
| Henbit | yes |
| Knawel | ?1 |
| Purple deadnettle | yes |
| Shepherdspurse | no |
| Sowthistle spp. | no |
| Swinecress | ?1 |
| Vetch spp. | no |
| Virginia pepperweed | no |
| Wild mustard | no |
| Wild radish | no |
| 1 ? Indicates that weed response is not known. | |
| Table 5. Trifluralin 4EC Rates for Canola. | ||
| Soil Texture | Rate (lb a.i./acre) |
Amount of Trifluralin 4EC/acre |
| Coarse* | 0.5 | 1.0 pt. |
| Medium** | 0.75 | 1.5 pt. |
| * Coarse soils are sands, loamy sands and sandy loams. ** Medium soils are loams, silty clay loams, silt loams, silts and sandy clay loams. |
||
Potential Weed or Herbicide Problems in Canola
Research is presently being conducted to identify effective herbicides and other strategies for weed control in canola. But it is difficult to predict all the weed or herbicide problems that may be encountered. The following weed control and herbicide facts are known:
- Follow all cultural recommendations to promote uniform emergence and growth of canola. Canola competition with weeds will help eliminate late developing weed problems.
- Trifluralin is highly recommended as a preplant incorporated herbicide.
- Do not plant fields infested with wild radish and wild mustard with conventional canola. Trifluralin will not control these weeds and there are no selective postemergence herbicides available. (Note: Wild radish and wild mustard may be controlled by using herbicide-tolerant canola varieties.)
- Use selective grass herbicides (clethodim, quizalofop or sethoxydim) as needed to control grassy weeds such as annual ryegrass.
- Canola can be severely injured by the following commonly used crop herbicides:
- 2, 4-D and other phenoxy herbicides
- acifluorfen (Ultra Blazer)
- atrazine (various trade names)
- bentazon (Basagran)
- chlorimuron (Classic)
- chlorimuron + metribuzin (Canopy)
- dicamba (Banvel, Clarity)
- diclosulam (Strongarm)
- fomesafen (Reflex)
- glyphosate (Roundup, others)
- glufosinate (Liberty)
- imazapic (Cadre)
- imazaquin (Scepter)
- imazethapyr (Pursuit)
- metribuzin (Sencor)
- metsulfuron (Ally, Cimarron)
- pyrithiobac (Staple)
- simazine (various trade names)
- thifensulfuron + tribenuron (Harmony Extra)
Many of the above herbicides are used in crops that will be growing adjacent to canola. Drift from ground or aerial applications can cause severe canola injury. Canola producers should use caution when applying these herbicides near canola and perhaps should also advise other farmers and aerial applicators of the sensitivity of canola to these herbicides.
- Volunteer Canola: Canola seed have the ability to persist in the soil, which may cause canola to behave as a weed in crops that follow canola. Based on its susceptibility to most crop herbicides, canola can be controlled, but producers should be aware that additional herbicide expenditures may be necessary in crops that follow canola.
- Rotational restrictions must be followed to avoid herbicide residue carryover problems from previous crops of corn, soybeans, peanuts or cotton. Table 6 shows the rotational restrictions of row crop herbicides that may cause carryover problems in a succeeding canola crop.
Preliminary results from Georgia research would suggest that Clearfield canola varieties could be planted in fields previously treated with Cadre (imazapic) or Staple (pyrithiobac) but not Strongarm (diclosulam).
Growers who intend to plant canola in the fall should consider using the following herbicides on labeled spring/summer planted crops: Cobra (lactofen), Dual Magnum (s-metolachlor), Liberty (glufosinate), Roundup (glyphosate), and Valor (flumioxazin). When applied to spring/summer planted crops, these herbicides pose minimal threat to fall-planted canola when used as labeled.
| Table 6. Rotational Restrictions of Herbicides that May Cause Carryover Problems in Canola. | |
| Herbicide | Canola Rotation Restriction |
| Atrazine1 | 12 months |
| Boundary | 12 months |
| Cadre | 40 months |
| Callisto | 10 months |
| Capreno | 18 months |
| Classic | 18 months |
| Cobra | 1 month |
| Envive | 18 months |
| Envoke | 18 months |
| Fluometuron (Cotoran, others) | 6 months |
| Halex GT | 10 months |
| Laudis | 10 months |
| Linuron (Lorox, others) | 4 months |
| Prefix | 18 months |
| Pursuit | 26 months |
| Reflex | 18 months |
| Sencor | 12 months |
| Spartan | 24 months |
| Staple | 10 months + FB3 |
| Steadfast Q | 10-18 months |
| Strongarm | 30 months |
| Valor | 4 months (tillage) 8 months (no-tillage) |
| Zidua | 18 months |
| 1 Research conducted in Tennessee and Georgia showed that canola could be planted in the fall following spring preemergence applications of atrazine on corn. Atrazine at rates up to 4 lb. a.i./acre were used in this study. The potential for atrazine to cause carryover injury to canola with growing seasons that have normal rainfall would be low. 2 FB - field bioassay. A successful field bioassay means growing to maturity a test strip of the crop intended for production the following year. |
|
Canola Diseases
Major Diseases
Canola, like all crops, is subject to diseases that are capable of reducing yield and quality if they are not controlled. In most situations and years, however, disease losses on canola are minimal if good management practices are used. Control of canola diseases in Georgia and the southeastern United States can be accomplished by following good management practices and selecting adapted, disease resistant cultivars.
Two major diseases of canola in Georgia are blackleg and Sclerotinia stem rot. Blackleg can kill plants from the seedling stage until maturity and can cause serious yield losses. Fortunately, blackleg resistant cultivars will provide excellent control when used with good management practices. Sclerotinia stem rot can kill plants during the rosette stage and during the flowering and pod-filling stages. Plants lost during the rosette stage may have little effect on yield if adjacent plants are able to compensate, but plants lost after flowering can cause yield reductions. Stem rot can be most effectively controlled by site selection and crop rotation in conjunction with other good management practices.
Blackleg
Blackleg, caused by the fungus Leptosphaeria maculans, is the most serious canola disease in most production areas worldwide. Blackleg was first observed on Georgia canola late in the 1992-1993 season at several locations. Blackleg was more common in the 1993-1994 crop with significant losses of susceptible varieties in some fields. The distribution of blackleg in Georgia during the late 1990s is shown in Figure 5.
The spores that cause infections arise from infested and infected seed and crop debris. Spores can be windborne for at least 3 miles from where they are produced. Seed sources are an important means by which blackleg is introduced into new production areas. Once the disease becomes established in an area, crop debris is the most important source of infections, and seed becomes less significant as an infection source. Debris from even the most resistant cultivars can be infected and serve as a primary infection for the next season.
Leaf infections appear as round to irregular shaped lesions. They are white to tan in the center and become papery, often breaking up as they age. They are almost always dotted to some extent with small black bodies called pycnidia. A minor disease, white leaf spot, produces lesions that are similar to and may be confused with blackleg lesions. White leaf spot lesions lack the definite border and do not develop the black dotted appearance characteristic of blackleg.
Blackleg infections of the stem and leaves can occur throughout the season. Disease losses occur when stem cankers develop at the crown of the plant weakening this area causing the infected plant to fall over.
Most crown rot results from fall infections occurring from the cotyledon to sixth to eighth leaf stage. These infections can remain dormant through the winter and become active during and after bloom, resulting in a girdling crown lesion on susceptible cultivars.
Cultivars vary from extremely susceptible to very resistant to blackleg. Resistant cultivars are often infected, but the girdling crown lesions do not develop on many of the plants. The percentage of plants that do not develop crown lesions under severe disease pressure is used as the measure of blackleg resistance. Leaf lesion numbers are not an accurate prediction of how much or if any crown rot and loss will occur. Leaf lesions are not especially good indicators for variety resistance either.
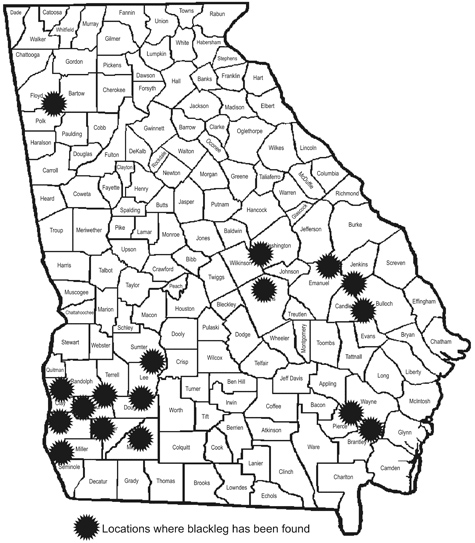 Figure 5. Distribution of blackleg in Georgia.
Figure 5. Distribution of blackleg in Georgia.

 Figure 6. Blackleg infection on canola stem showing pycnidia (top), and stem with girdling crown lesion (bottom), causing plant to break off at ground level.
Figure 6. Blackleg infection on canola stem showing pycnidia (top), and stem with girdling crown lesion (bottom), causing plant to break off at ground level.
The most important means of blackleg control is through the use of resistant cultivars. The other recommended disease control practices listed in the next section on Basics of Disease Control are important and are aimed at keeping disease pressure as low as possible on whatever level of cultivar resistance is selected.
Sclerotinia Stem Rot
Sclerotinia stem rot, also known as white mold, is caused by the soil-borne fungus Sclerotinia sclerotiorum. It has a wide host range and can attack almost any broadleafed plant, but it is not a problem on grasses. This fungus is active only during cool, wet weather. In Georgia, the fungus is active only during the winter months and has never been a problem on any of our summer crops, although it has been present in the soil throughout Georgia for many years. Infections on canola have not been observed before December, and new infections are rarely seen after April.
The fungus survives the hot summer months as sclerotia in the soil. Sclerotia can survive for a year in southern Georgia soils and for up to 2 years in northern Georgia with no decline in viability. Viability declines over subsequent years. Any time during the winter, if the soil becomes very wet for several days, sclerotia can germinate in two ways: by producing mycelium in the soil or by producing small mushroom-like apothecia that produce spores above the soil surface. These spores are disseminated by wind and rain to other parts of the field or to adjacent fields.
Neither the mycelium nor the spores can invade a healthy canola plant directly. Both must become established in dead organic matter in contact with healthy tissues to initiate disease. Once a disease lesion is initiated, the fungus secretes acids and enzymes that kill additional plant tissues. The fungus can rapidly destroy small plants or girdle the stem of large plants if the weather remains wet and cool.
During the rosette stage, disease lesions usually begin in areas of dead leaf tissue that come in contact with mycelium growing in the soil. Freeze injury to leaves is often a source of this dead leaf tissue. Rosette stage infections often start on one leaf, and the white, fluffy fungus quickly grows to the crown and outward on the other leaves. Surrounding plants may be infected if their leaves are in contact, creating roughly circular areas of dead or dying plants. During the flowering period, most lesions are initiated when spores land on shed flower petals that adhere to leaves or stems.
 Figure 7. Sclerotinia infection in rosette stage plants.
Figure 7. Sclerotinia infection in rosette stage plants. Figure 8. Sclerotinia infected stem during flowering.
Figure 8. Sclerotinia infected stem during flowering.
 Figure 9. Sclerotinia infection (white portion of stems) in canola during seed fill.
Figure 9. Sclerotinia infection (white portion of stems) in canola during seed fill. Figure 10. Sclerotia (dark objects) on soil surface.
Figure 10. Sclerotia (dark objects) on soil surface.
After bolting, infections appear as soft, water-soaked areas on the stem. These lesions become light brown. As they expand up and down the stem, they become gray with faint concentric markings. In wet weather, or deep in the canopy where drying is slow, the lesions may be covered by white fungus growth. As the lesions age, the stems may appear soft and often mushy when wet, but somewhat shredded under dry conditions.
Infected plants eventually die, and the lesion takes on a distinctive bleached appearance. Infected stems can break off at the lesion and collapse before harvest. The stems of infected plants will be found to contain one to several hard, somewhat irregularly shaped black bodies 1/8 to 1/2 inch long. These are the sclerotia that are responsible for survival of the pathogen from one season to the next.
There are no canola cultivars with resistance to stem rot and, in very wet seasons, some plants may be killed in any field in Georgia. In drier years, fewer plants will be killed by this disease. Poorly drained fields suffer losses much more frequently than well-drained fields. The most important control measure is to select well drained fields that have not had canola or another susceptible crop for the past two winters.
Minor Diseases
Seedling Diseases. If high quality seed treated with a fungicide is planted at the proper depth in a well prepared, firm, moist seed bed during the recommended planting period, seedling diseases are usually not a significant problem. However, if the soil is poorly prepared, very wet or very dry, or excessively hot or cold, or if the seed is planted very deep, seedling diseases can reduce stands.
The fungicide seed treatment can provide some protection during germination but will not protect the seedling after emergence. The best control measure is to avoid planting when these adverse conditions exist.
Alternaria Black Spot. Alternaria black spot is a disease of leaves, stems and seed pods on canola. It is caused by more than one species of Alternaria. In Georgia, A. brassicae and A. brassicicola have been found on canola. A. brassicae is favored by cooler temperatures than A. brassicicola. Alternaria leaf spots vary from gray to gray with purple borders to black depending on environmental conditions. The leaf spot phase is usually harmless but may serve to build up the fungus for later stem and pod invasions. Stem and pod lesions begin as small dark dots. These dots expand to form circular lesions or, at times, large irregular diseased areas. As the crop matures, heavily infected pods take on a gray sooty look compared to a golden brown of healthy pods. Infected pods commonly contain shriveled seed and may split open early resulting in further loss from seed shatter.
White Leaf Spot. White leaf spot is caused by the fungus Pseudocercosporella capsellae. The symptoms are light (approaching white) papery lesions with irregular borders. There may be a diffuse, sparse black growth of fungus material in the lesions. White leaf spot should not be confused with leaf lesions caused by the blackleg fungus, which have more regular borders and are dotted with black fungus bodies erupting through the leaf tissue. This disease has not been severe on cultivars of B. napus in Georgia.
Black Rot. Black rot is a common seed-borne disease of cabbage and other crucifers. Black rot is caused by a bacterium, Xanthomas campestris. The disease causes yellow wedge-shaped spots extending in from leaf margins. Black rot bacteria can remain active in no decomposed plant litter for up to two years. Black rot has not caused major losses in canola.
Downy Mildew. Downy mildew, caused by Peronospora parasitica, results in white to light gray fungus growth on lower leaf surfaces. There may be some pale purple marks associated with these spots. Upper leaf surfaces show distinct yellow spots. Downy mildew is most active on canola in mild wet weather. It does not appear to result in yield loss in Georgia.
Nematodes. Root-knot nematodes (RKN) are capable of invading and reproducing on canola. Greenhouse studies indicate that cultivars of B. napus vary in reaction to RKN but that this species is generally a poor host for these nematodes. Field studies indicate that RKN do not consistently cause measurable damage to canola, and that RKN populations do not consistently increase on canola. This is probably a result of a rather poor host being grown during the winter season when low soil temperatures greatly reduce the activity of RKN.
Basics of Disease Control
The following good management practices will greatly reduce the probability of losses from canola diseases.
Site Selection and Crop Rotation. Select fields that are well drained and have not had canola, a winter legume or a crop of cabbage, collards or turnips for the past two winters. Keep canola crops as far apart in distance and time as possible. This is important in preventing damage from Sclerotinia stem rot and blackleg. Avoid fields with a major problem with winter leguminous weeds or wild radish during the past two winters.
Cultivar Selection. Select cultivars of Brassica napus that have been developed and/or tested for production in the southeastern United States. Be certain that the cultivar has sufficient resistance to blackleg for production in your immediate area. If possible, chose a cultivar with the highest resistance level. Do not plant a susceptible canola within 3 miles of a field previously infected with blackleg.
Seed and Seed Treatment. Plant only high quality seed that has been treated with a fungicide to help prevent seedling disease problems and help delay the introduction of blackleg into new production areas. Contaminated seed can introduce blackleg into new production areas and can also introduce new blackleg races into established production areas. Seed treatment is very (but not totally) effective in controlling blackleg where seed is the source of the fungus. Seed treatment will not protect germinated seedlings from infection where old litter is the source of the fungus.
Land Preparation and Planting. Plant the seed at the recommended depth into well prepared, firm soil. Plant only during the recommended planting period for your area. Avoid planting when the soil is excessively wet or dry. This will help prevent seedling disease and improve stand establishment. Planting outside the recommended planting period increases the risk of cold injury, which can greatly increase the incidence of Sclerotinia stem rot.
Destroy Canola Residue. Destruction of canola residue as soon as possible after harvest will reduce the disease intensity in future canola crops. Bush-hogging or any tillage operation that reduces the size of the pieces of canola debris and buries them in the soil will hasten the decomposition of the residue. Rapid decomposition of residue will help reduce both blackleg and Sclerotinia stem rot in future canola crops.
Scouting for Diseases. Scouts should check for blackleg and Sclerotinia stem rot while scouting fields. There is little that can be done, and normally nothing that needs to be done, to control diseases after planting. Information on disease incidence will determine if the cultivars you are planting have sufficient blackleg resistance and also help identify fields that need a longer rotation or should be avoided in the future to reduce damage from Sclerotinia stem rot. Several foliar fungicides are labeled for use on canola. Fungicide application during early flowering can be effective in reducing yield losses associated with late-season Sclerotinia and other fungal diseases if production economics allow for this expenditure.
Insect Pests of Canola
Insects can significantly reduce canola yield. Growers must be prepared to scout and prevent injury from insects in canola. Specific insecticide recommendations are updated annually and are available in the Georgia Pest Management Handbook, commercial edition, at http://www.ent.uga.edu/pmh/Com_Canola.pdf
Major Insect Pests
Aphids
Of all the insects attacking canola, aphids have been the most damaging to the crop in Georgia. Three aphids have been identified and found to damage canola: the turnip aphid (Lipaphis erysimi), cabbage aphid (Brevicoryne brassicae) and green peach aphid (Myzus persicae). Turnip aphids are bluish-green (Figure 11). Green peach aphid color varies from medium green to yellow and, under cool temperatures in the winter, reddish-brown. Cabbage aphids are a mediumgray. Mild weather conditions in the fall and early spring can allow aphids to explode to very high populations.
Canola damaged in the seedling stage appears stunted and is more susceptible to winterkill. Damaged seedlings will have curled leaves with shortened nodes.
The canola may exhibit a purplish tint associated with plant stress. Stunting and slow recovery is a response to toxins injected by the aphids during feeding. Stunted plants often remain smaller than normal plants throughout the growing season, even after the aphids have been controlled (Figure 12). Aphid damaged plants never produce to their maximum levels. Aphids commonly cause yield losses of 10 to 15 bu/acre.
Aphids are most obvious on canola when feeding on flower heads (Figure 13). This is often the time when growers first realize that they have an aphid infestation. As the heads emerge and bloom begins, aphids will concentrate on the heads, where they cause the stems to appear black and podless. The black appearance is caused by sooty mold. Aphid populations that develop during early bloom and pod-fill can cause deformed, stunted or completely sterile pods. Aphid populations typically decline and disperse after bloom and do not cause yield losses during the pod-fill stage.
Several natural enemies help to regulate aphid populations. The most important are a parasitic wasp and predatory ladybird beetle larvae and adults. The figure of aphids on stems with pods shows lady beetle larvae. These natural enemies can kill large numbers of aphids. They are most active in the fall before frost and in the spring during the bloom period; they are not active during the winter months, consequently aphid populations can increase unchecked during warm periods in the winter.
Aphids can be controlled with insecticides. Canola should be scouted biweekly for aphids. Treat for aphids when populations exceed two per plant in the seedling stage, five per leaf in the rosette stage, or when 20 percent of the heads are infested during bloom. Insecticide seed treatments, such as imidacloprid (Gaucho, others), are available that suppress aphids for several weeks after planting. After this time, usually one well-timed application will control aphids, although two applications may be needed in some fields. In Georgia, the largest increase in yield occurs when aphid populations are controlled during the rosette stage in mid to late winter before bloom. Do not treat late-blooming canola for aphids because populations usually decline after bloom. Treatment of flowering canola for aphids is very hazardous to honey bees and should not be done unless absolutely necessary to prevent crop loss.
Aphid-Transmitted Viruses. Aphids, primarily green peach aphid and cabbage aphid, have been reported to transmit several viruses to canola. Viruses include turnip mosaic, cauliflower mosaic, and beet western yellows and broccoli necrotic yellows viruses. Serious yield loss has been reported from these viruses in some areas of the world. None of these viruses have been found to be prevalent in canola in Georgia. Currently, aphid-transmitted viruses are not considered to be a significant problem in canola in Georgia.
 Figure 11. Turnip aphids on canola leaf.
Figure 11. Turnip aphids on canola leaf. Figure 12. Aphid damaged area in field during bud/early bloom stage. Aphid injury during the rosette stage stunted and killed plants.
Figure 12. Aphid damaged area in field during bud/early bloom stage. Aphid injury during the rosette stage stunted and killed plants. Figure 13. Aphids on canola stalk causing deformed and aborted pods. Note seven-spotted lady beetle larvae feeding on aphids.
Figure 13. Aphids on canola stalk causing deformed and aborted pods. Note seven-spotted lady beetle larvae feeding on aphids.Cabbage Seedpod Weevil
The cabbage seedpod weevil (Ceutorhynchus obstrictus) is the most damaging insect pest of canola in north Georgia. Infestations in the Limestone Valley and northern Piedmont regions reduced yield by 60 percent in 1991. Damaging infestations were not present in southern Georgia (zone 8 in Figure 3) until recently, but significant damage was observed in this area in spring of 2007. In areas of the northern United States and Canada where this insect occurs, treatments for cabbage seedpod weevil control are made annually.
The adult weevil is small, ash-grey in appearance and measures approximately c inch long (Figure 14). It has a snout, which is typical of the weevil family. One generation develops per year. Adults emerge from overwintering sites in field edges and wooded areas and enter fields as the canola begins to flower. Female weevils puncture a hole in the developing canola pod and lay a single egg. Weevils lay from 25 to 50 eggs. Eggs hatch in 5 to 30 days into white legless larvae. Each larva may consume 5 to 8 seeds in a pod (Figure 15). Typically, one larva occurs per pod, although when infestations exceed 50 percent infested pods, two or three larvae per pod are common. Mature larvae bore a hole in the canola pod and fall to the ground to pupate. Pupation lasts from 11 to 28 days. Newly emerging adults climb back onto the canola where they feed on stems and pods before dispersing. New adults often puncture and feed on seeds in green pods, which reduces seed size and quality and ability of seed to germinate properly.
Prior history of weevil is the primary consideration in determining if a weevil treatment is necessary. If weevils occur in the area and canola has been grown for more than 7 years, an insecticide treatment for weevils can be expected. A single application of insecticide at about 75 percent bloom has been found to be most effective in controlling cabbage seedpod weevil. Treat for cabbage seedpod weevil when an average of two or more weevils are found per plant at 50-75 percent bloom. In a few instances, a second application may be necessary. A second treatment should be made 7 to 10 days after the first treatment if weevil populations average one per plant. Check insecticide labels, because some products have restrictions on the interval between treatments.
 Figure 14. Cabbage seedpod weevil on a canola flower.
Figure 14. Cabbage seedpod weevil on a canola flower. Figure 15. Canola pod with top removed to show seed damage by cabbage seedpod weevil larva. Undamaged seed on the right.
Figure 15. Canola pod with top removed to show seed damage by cabbage seedpod weevil larva. Undamaged seed on the right.
Minor Insect Pests
Turnip Root Aphid. Also known as the popular petiole gall aphid, the turnip root aphid infests and feeds on roots of Brassica plants such as turnips and canola during the fall, winter and spring. The aphid moves to popular trees during late spring, where they form galls on leaf petioles during the summer months. When feeding on canola roots, they produce a waxy coating that is easily seen when roots are exposed and may sometimes be seen on the soil surface above infested roots. Turnip root aphid feeding can reduce plant growth and vigor, but injury usually is not apparent. Turnip root aphid is an occasional pest of canola and infestations often are very spotty in distribution. Management thresholds are not available, although insecticide seed treatments may help suppress infestations in the fall.
Foliage Feeders. The most important leaf feeders are the diamondback moth (Plutella xylostella), cabbage looper (Trichoplusia ni) and cabbageworm (Pieris rapae). Of these, diamondback moth has the greatest potential to cause damage (Figure 16). It can be found infesting canola throughout the growing season, feeding on foliage. Defoliation can be especially severe if it occurs just prior to pod development. Consider treatment if defoliation by all foliage feeders exceeds 15 percent of leaves. Diamondback larvae also will chew holes in flowers and green pods. Most canola-feeding caterpillars also feed on other crops, with diamondback moth being a severe pest of other leafy vegetables in Georgia.
Lygus and Plant Bugs. Lygus bugs (Lygus spp.) infest canola during bloom and green pod stage. Adults (Figure 17) and nymphs occur with one generation usually completing development in canola before pod maturity. Feeding can deform and blast flowers. Feeding also will shrivel seed and cause a feeding blemish on green pods. Consider an insecticide treatment if populations average two or more bugs per sweep. Because canola blooms early in the spring, Lygus bug numbers usually do not exceed the treatment threshold. However, canola can serve as an early season host for Lygus bugs, thereby allowing them to build up and disperse to summer crops as canola matures.
 Figure 16a. Diamondback moth larva.
Figure 16a. Diamondback moth larva. Figure 16b. Diamondback moth pupa.
Figure 16b. Diamondback moth pupa.
 Figure 17. Lygus bug adult on canola flower buds.
Figure 17. Lygus bug adult on canola flower buds.Flea Beetles. Flea beetles (Phyllotreta spp.) are the most important pest of spring-planted canola in the northern Great Plains and Canada, where adult flea beetles feed on and destroy seedling stands. In the southeastern United States, where canola is fallplanted, flea beetle is a minor pest. Occasionally flea beetles may damage seedling stands in the fall, but more often they are present in the spring when they can cause small, shot-hole type defoliation during bud and bloom stage. Canola is tolerant of this defoliation, however, if it does not exceed 15 percent of the leaf area. Flea beetle larvae occur in the soil where they feed on canola roots. Larval feeding is not considered to be important.
Pollen Beetle. Pollen beetles (Meligethes sp. near M. nigrescens) are common on flowers of canola. Pollen beetles in Georgia are not the same species as those in Europe, where pollen beetle is an important pest. Adults are about 1/8 inch long, solid black with clubbed antennae and chewing mouthparts. Pollen beetles feed on flower pollen but are not known to cause yield losses in canola in Georgia.
False Chinch Bugs. False chinch bugs (Nysius raphanus) typically infest canola during late bloom and pod fill stages. Populations can become very large immediately before harvest. Adults are about 5 mm long and uniformly gray. Nymphs and adults suck sap from the plant. Heavy or prolonged feeding injury can reduce pod set and seed fill. However, infestations only occasionally reach damaging levels in Georgia. Treatment threshold is 25 or more bugs per sweep. In double-crop and reduced tillage situations, false chinch bugs may remain in the same field after harvest and cause damage to emerging seedlings of the next summer crop following canola.
Bird Damage. As canola matures, birds may sometimes land on mature seed pods and feed on seed. Their feeding activity often shatters pods resulting in more seed loss than direct feeding. Bird damage usually is minimal in large fields and confined to field edges or places in the field where birds can roost.
Bees and Canola
All canola grown commercially in the south is Brassica napus, which is self-fertile and mostly selfpollinated. Each flower has both male and female parts, and the crop is a rich source of pollen and nectar for bees and other insects. Flowers open at any time of day. Wind usually is all that is needed for pollination. In fact, canola in field plots caged to exclude insects often set seed as well as non-caged plots. In contrast, plants grown in still, insect-free greenhouses typically have poor seed set. Insects are helpful supplemental pollinators and often will shorten the time needed for pollination and seed set. Normally, however, bees are not needed for successful pollination and seed set of B. napus canola.
 Figure 18. Honey bee foraging on canola flowers.
Figure 18. Honey bee foraging on canola flowers.Nevertheless, flowering canola is highly attractive to pollinating insects; few other crops promote such intense bee visitation. In Georgia, pollinators of canola include, honey bees, bumble bees, large carpenter bees, and other native, solitary bees such as andrenid and halictid bees. At the time of spring when canola flowers, it is one of the few nectar sources for honey bees. Since bees can only help pollination, growers should do nothing to reduce bee populations, such as apply insecticide while canola is blooming.
Because canola is an early, rich source of nectar, beekeepers may ask to locate hives in canola during bloom. Honey yield potential can range from 90 to 450 pounds per acre in Europe, although in the United States, beekeepers more commonly measure yield per hive. Georgia beekeepers report about 35 to 70 pounds per hive. The quality of canola honey is poor by southern U.S. standards. It has a light color and mild flavor, but it granulates extremely fast and shelf life of bottled canola honey is relatively short. Canola pollen and honey are best used as bee feed to speed colony growth for later spring activities.
Harvesting and Handling Canola
Harvesting
When to Harvest
Canola ripens quickly, making timely harvest extremely critical for maximum yield. The seed reaches physiological maturity at about 40 percent moisture content and begins to turn from green to yellow, brown or black. Once the pod is filled, seeds dry rapidly at a rate of 1 to 3 percent per day depending on weather conditions. Harvesting too early results in too many green seeds and light test weight, while late harvesting can result in excessive shattering and yield loss. Late harvest also increases the potential for weather related delays and for lower soybean yields for double-crop producers. Direct combining and swathing are widely used for harvesting canola in the most areas.
The number of green seeds in the field, the moisture content of the canola seed, and the presence of dew or surface moisture on the plant should be monitored to determine when the crop is ready to combine. Green seed should not exceed 2 percent when marketed. Two green seed per 100 produces oil with an undesirable green color and leads to dockage in grade and price. Direct combining should begin when seed moisture content reaches 8 to 10 percent and the seed turn dark (few or no green seeds). Canola is generally sold without dockage when seed is 9 percent moisture or less.
To test for the number of green seeds, gather a total sample of 100 seeds from three to four locations in the field. Place them on the sticky side of a piece of tape to hold them in place. Press the tape with the sticky side down and crush the seeds with a wooden rolling pin. Count the number of seeds with a distinctly green interior. If this number is more than two (2% of your sample), wait a day or two and make another count. Since canola ripens from the bottom of the stalk up, check every two to three days after some color change is noted in the pods on the bottom of the main stem.
Swathing
Historically, canola grown in Georgia has been harvested by direct combining without prior swathing. However, if the proper equipment is available, canola can be swathed when the crop reaches 35 percent moisture content in the field. A visual indication of this condition is when about a third of the seeds have turned a dark brown color. Reserve nutrients in the stem and leaves are sufficient to complete seed development.
The swath should be placed on top of the stubble where it will dry in approximately one to two weeks or even sooner under hot, dry weather. When the seed has dried to 9 percent, the crop can be threshed and stored. Other suggested guidelines for swathing include:
- Check the operator's manual for specific machine settings.
- Swath the crop when 30 to 35 percent of the seeds on the main stem only have changed color.
- Set the cutter bar about 8 to 12 inches above the ground or just below the bottom seed pods.
- Swathers should have a table depth of 39 inches or more, a throat width of 39 to 51 inches, and a vertical clearance of 29 to 39 inches to handle the bulk.
- Crop dividers or vertical knives may be required in a heavily lodged crop.
Direct Combining
Direct harvesting can begin when crop moisture has reached nine percent. Conventional and rotary combines are effective for harvesting canola if properly adjusted. Ground speed usually is in the 2-3 mph range for good uniform stands.
Refer to the operator's manual for specific machine settings for canola (or rapeseed) prior to going to the field. In absence of specific information in the operatorâ??s manual, the following settings may be used as a starting point.
- Rotor/cylinder speed: 450 to 600 rpm
- Concave clearance:
- Conventional: 1/2 to 1 inch front, 1/8 to 3/4 inch rear
- Rotary: 1/2 to 1 1/2 inch
- Fan speed: 400 to 600 rpm
- Chaffer setting: 1/4 to 3/8 inch
- Cleaning sieve: 1/8 to 1/4 inch
From this starting point, use experience and field conditions to make further adjustments.
Machines with rigid cutter bars should have the cutting height set just below the seed pods. Cutting too low on the plant stem can reduce ground speed, increase surface moisture on the seed and unnecessarily increase the amount of material that must be handled by the machine.
The reel should be set high initially and as far back over the table as possible. In the field, lower the reel slightly toward the crop just until smooth feeding is accomplished. Match reel speed to ground speed to minimize shatter.
Cylinder or rotor speeds should be set at about 1/2 to 2/3 that used for wheat, depending on crop conditions. Under proper conditions, the speed should be set just fast enough to break open the pods. Reduced speeds are important to prevent over-threshing pods and stems and overloading the sieves.
Set the concave clearance to minimize the amount of broken material (pods and stems) passing over the sieves that interferes with seed separation. A 3/8- to 1/2-inch clearance should be sufficient to minimize losses. Open the front concave clearance fairly wide (up to 1 inch) if excessive losses occur.
Few adjustments can be made to straw walkers to improve seed separation. Unthreshed pods, broken pods and straw returned from the straw walkers tend to overload the sieve and return augers. Installing a 1/4 screen over a portion of each straw walker tends to help improve separation.
The chaffer (top sieve) should be opened enough for good separation. Unlike other grains, the cleaning action for canola should rely more on a shaking action for separation and less on air separation. Air should lift the chaff on the sieve while the shaking action conveys the material rearward. Canola seeds are light and are easily blown out the back of the combine if the airflow rate is too high. Start with a low fan speed and gradually increase it until good separation occurs with no canola being blown over the chaffer. If canola is lost out the rear of the combine with the fan and chaffer opening balanced, control motor speed and ground speed to reduce grain losses. Generally, itâ??s better to control air volume by manipulating fan speed than by adjusting chaffer vanes.
Machine Adjustments in the Field
Field conditions change hourly, usually changing combine performance. Frequent checks and readjustments may be necessary in the field if harvest losses are to be held to acceptable levels. The amount of trash in the harvested crop can be reduced by increasing fan speed, closing the bottom adjustable sieve, and/ or opening the concave slightly. Check for leaky grain points from auger housings or pans. Use duct tape or caulking to seal loose or rusty conveyor housings.
A combination of adjustments may be needed to avoid overloading the lower sieve. If returns are too high, try the following adjustments in order: (1) close the chaffer, (2) open the shoe, (3) reduce fan speed and (4) reduce the cylinder/rotor speed or ground speed.
Harvest Losses
Incorrect settings can lead to losses, so frequent checks and adjustments are desirable. Common seed loss rates are 25 to 50 pounds per acre (1/2 to 1 bushel). This corresponds to a loss of 50 to 100 seeds per square foot.
Harvest-Aids/Desiccants
Reglone 2SL (diquat) can be used as an harvest aid/ desiccant to speed up the drying process. Reglone should be applied when the canola is in the 60-75% seed turn stage (green to brown) and harvested 7-10 days after application.
Drying and Storing
Storage Structures
Canola stores best when held in weather-tight, rodentproof structures. Round metal bins are the best choice, assuming all leaks are sealed and access by mice, rats and birds is eliminated. Bins without perforated floors should be sealed along the foundation ring to prevent moisture uptake by the grain on the floor.
Put only clean canola into bins for storage. The perforated floor should be covered with plastic or wire window screen to contain the seed. Canola seeds are very small (0.05 to 0.09 inch diameter, depending on variety). Fines generally collect 3 to 4 feet from the center of the bin floor rather than a center cone like other grains. Grain distributors have generally not been effective and so only clean seed should be stored. It is especially important to level the grain surface after binning canola. This helps eliminate heat build-up in the peak and aids in uniform airflow through the top surface.
Temperature monitoring equipment is recommended to check the condition of canola during storage. Temperatures should be checked frequently (every two days) after initial binning and aeration to be sure conditions have stabilized. Observations may be less frequent (every one to two weeks) after the canola has been cooled or turned (moved to another storage bin).
If monitoring equipment or temperature cables are not available, core the bin within a few days after storage and check for signs of heating and spoiling. Storage managers report that canola has a distinctive odor when it is going out of condition. If unusual odors are detected, the canola should be aerated or turned immediately and sampled thoroughly to determine the extent of damage.
Handling and Cleaning
Most common handling equipment can be used for loading and unloading canola into or out of trucks, wagons and bins. The diameter of a canola seed ranges from 3/64 to 3/32 inch, so the slightest cracks in boots, drop boxes and enclosures of transporting equipment must be sealed with duct tape or caulk.
Operate augers at full capacity and moderate speeds to avoid excessive damage to the seed. Conveyor belts should be used where available provided steep inclines are avoided and discharge chutes are positioned directly over the belt to lay the seed in the direction of flow. Drag conveyors have been used successfully, but experience with canola suggests that the spacing between paddles should be reduced to maintain satisfactory capacity. Pneumatic conveyors can handle canola adequately but may have difficulty in feeding and discharging the seed. Kernel damage is usually not a problem for most equipment unless the moisture content is below 7 percent.
All canola lots contain certain levels of fines or trash and chaff. Uneven distribution of trash and fines in a drier or storage bin causes uneven airflow through the grain. Trash and fines create higher resistance to airflow than clean canola and reduce the drying or cooling rate.
Mechanical cleaners generally work better than pneumatic cleaners for canola, but the cleaning capacity may be 5 to 10 percent lower than for other grains. Since most of the trash is larger than canola seeds, screens and scalpers work well. Cleanings should be burned or spread back in the field so as not to provide a good harbor for insects near the storage bins.
Fans and Airflow
Resistance to airflow for canola is 6 to 10 times that of corn and 2 to 3 times the resistance of wheat. Consequently, airflow through canola is very restricted as compared to other grains. Expect even the best equipment to be severely limited in the amount of air that can be provided for canola drying and aeration. Air delivery and efficiency of drying or aeration fans can best be enhanced by applying the following rules:
- use large diameter bins,
- keep grain depth shallow,
- level the top surface and
- use centrifugal fans.
Bin drying fans should provide at least 1 cubic foot of airflow per minute (cfm) per bushel of undried grain. Aeration fans should provide at least 1/10 and preferably 1/4 1 cfm per bushel of grain to be cooled. Approximate airflow capabilities for common axial and centrifugal fans are shown as functions of grain depth. Figure 19 illustrates how air delivery capability of axial and centrifugal fans is affected by canola depth in a bin. Most axial fans would not provide minimum drying airflow (1 cfm/bu) if grain was more than 7 feet deep. At the same 7-foot depth, a centrifugal fan could provide about 2 cfm per bushel. As a result, a centrifugal fan would reduce drying time for the 7 foot depth by about 50 percent as compared to an axial fan.
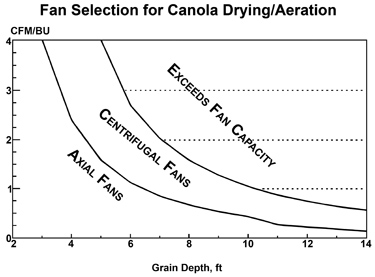 Figure 19. Fan selection for canola drying/aeration. (S. McNeill and D. Overhults. 1992.)
Figure 19. Fan selection for canola drying/aeration. (S. McNeill and D. Overhults. 1992.)Centrifugal fans could also provide the minimum air flow for drying at depths up to about 10 feet. Axial fans are generally adequate for aeration until canola depth exceeds 16 feet. All fans, axial or centrifugal, must have a total air delivery capability adequate for the total number of bushels of grain in the bin. An under-sized centrifugal fan should not be considered as an adequate replacement for a properly sized axial fan.
Aeration and Temperature Control
Cool canola immediately after drying to reduce the storage temperature as much and as quickly as possible. Cooling times are considerably slower for canola than with other grains at the same depth. If dry canola is placed directly in the bin from the field, run the fan for several days to remove field heat and to control heating by respiration. Stop the fan only after all the grain has been cooled. Check the grain below the surface a few days afterward to be sure no hot spots have developed. If you find hot spots, operate the fan regardless of the outside air conditions until these areas have cooled. In severe cases, the grain may need to be moved to another bin to disperse pockets of wet grain, trash or fines that can cause blockages to air flow.
A minimum rate of 1/10 cfm for each bushel (cfm/bu) is 1 usually adequate for aeration, provided that fines and trash are spread evenly throughout the bin. Compensate for the high resistance to air flow by reducing the depth to about half that for wheat or a third that for corn. At the minimum air flow rate, about 120 to 150 hours of continuous fan operation are required to force a cooling cycle completely through a bin of grain. If the rate is increased to 1/2 cfm/bu, cooling time should be between 24 and 30 hours.
Intermittent fan operation may be desirable at the larger air flow rate to take advantage of more favorable weather, but his requires a different type of fan and a much larger fan motor. For example, a 24-foot diameter bin with a 16-foot eave height (six rings) that is level full with canola requires a 1/2-horsepower motor on an axial fan to provide 1/10 cfm/bu. In contrast, if the farmer wanted to supply 1/2 cfm/bu, a 3-horsepower motor would be needed on a centrifugal fan.
Drying
Canola harvested above 10 percent moisture should be dried to 9 percent or lower, depending on the anticipated storage period. Expect lower air flows in drying bins or high speed dryers because of the smaller seed. So lower drying temperatures are recommended to avoid overheating the grain. If air blockage is coupled with excessive drying air temperatures in a high-speed dryer, fires may result. Keep dryer temperatures below 150 degrees F to avoid overheating problems. Cleaning the canola prior to drying also reduces the risk of fires. A maximum of 105 degrees F should be used for seed canola, since excess heat exposure lowers germination.
Batch and continuous flow dryers work well for canola, but some adjustments are needed. First, check the screen size on column dryers since most are equipped with a standard size of 3/32 inch. Since most canola seed varieties pass through or lodge in standard screens, smaller screens are mandatory. While manufacturers offer 0.04- to 0.07-inch screens for canola, most producers simply line the interior of their drying columns with window screen to avoid leakage problems without causing severe air flow restrictions. Check with the dryer manufacturer before making any changes to be sure fan operation will not be adversely affected. It is also a good idea to inspect floors and unloading augers and seal all cracks and crevices to prevent leakage of the tiny seeds.
The maximum drying air temperature for canola should be 150 degrees F (105 degrees F for seed canola), with a moisture content of 10 to 17 percent. Expect drying capacities to be about half the rated capacity for corn. Drying canola from 15 percent moisture to 10 percent is similar to drying wheat from 19.5 percent to 14.5 percent in terms of capacity and drying times.
Use alternate drying methods for very wet crops (>17 percent moisture content). One method is to run the canola through the dryer in more than one pass to avoid seed shriveling or cracking, and to maintain acceptable dryer performance. Reduce the drying air temperature by 10 degrees F for every 2 percent increase in moisture above 17 percent. A maximum moisture removal of 5 points for each pass through the dryer is suggested.
Molds can develop on canola if moisture is more than 90 percent. Periodically, operating fans to remove any moisture that may have accumulated after drying will reduce the chance of mold growth. Mites are a possible storage pest. Heavy infestations will leave a distinct odor. Mites can survive if moisture is 8 percent or more. Thoroughly clean bins before seed is stored.
Canola Production Costs and Marketing
Canola is not a capital intensive crop. Its production costs are less than those of many crops grown in Georgia and are likely to be similar to or slightly higher than winter wheat.
An important consideration in producing canola is the ability to sell it at a convenient local market for a good price. Producers should locate a buyer before committing to produce the crop and find out how canola will be priced.
The budget and tables beginning on page 25 provide a guide to the approximate costs of producing canola in Georgia. The cost items are estimates of the value of inputs needed to produce at the stated yields. Costs and yields vary across the state. Use these budgets as a guide. Substitute your own costs and practices into the budget to customize it for your operation.
* Section adapted from Harvesting, Drying and Storing, 1992 Canola Production and Management. University of Kentucky College of Agriculture — Cooperative Extension Service. ID 114.
| Canola, Dryland — Georgia, 2007 | ||||||
| Estimated Costs and Returns | ||||||
| Expected Yield: 40 bushels | Yield, Your Farm: | |||||
| Variable Costs | Unit | No. of Units |
$/ Unit |
Cost/ Acre |
$/ Bushel |
Your Farm |
| Seed | lbs. | 4.00 | $3.00 | $12.00 | $0.30 | |
| Lime | ton | 0.25 | $30.00 | $7.50 | $0.19 | |
| Fertilizer | ||||||
| Nitrogen | lbs. | 150.00 | $0.48 | $67.50 | $1.69 | |
| Phosphate (P2O5) | lbs. | 40.00 | $0.34 | $12.80 | $0.32 | |
| Potash (K2O) | lbs. | 40.00 | $0.24 | $9.60 | $0.24 | |
| Boron | lbs. | 1.00 | $3.75 | $3.75 | ||
| Sulfur | lbs. | 30.00 | $0.05 | $1.50 | ||
| Weed Control* | acre | 1.00 | $4.00 | $4.00 | $0.10 | |
| Insect Control | acre | 1.00 | $6.00 | $6.00 | $0.15 | |
| Machinery: Preharvest | ||||||
| Fuel | gallon | 3.23 | $2.25 | $7.28 | $0.18 | |
| Repairs & Maintenance | acre | 1.00 | $6.14 | $6.14 | $0.15 | |
| Machinery: Harvest | ||||||
| Fuel | gallon | 2.55 | $2.25 | $5.73 | $0.14 | |
| Repairs & Maintenance | acre | 1.00 | $3.92 | $3.92 | $0.10 | |
| Labor | hours | 0.74 | $10.00 | $7.36 | $0.18 | |
| Crop Insurance | acre | 1.00 | — | — | — | |
| Land Rental | acre | 1.00 | — | — | — | |
| Interest on Operating Capital | percent | $77.54 | 8.00% | $6.20 | $0.16 | |
| Drying - 2 points | bushel | — | — | |||
| Total Variable Costs | $161.28 | $4.03 | ||||
| Fixed Costs: | ||||||
| Machinery: Depreciation, Taxes, Insurance and Housing | ||||||
| Preharvest | acre | 1.00 | $16.88 | $16.88 | $0.42 | |
| Harvest | acre | 1.00 | $22.78 | $22.78 | $0.57 | |
| General Overhead | % of VC | $161.28 | 5.00% | $8.06 | $0.20 | |
| Management | % of VC | $161.28 | 5.00% | $8.06 | $0.20 | |
| Owned Land Costs: Taxes, Cash Payments, etc. |
acre | 1.00 | — | — | — | |
| Other | ||||||
| Total Fixed Costs | $55.78 | $1.39 | ||||
| Total Costs and Profit Goal | ||||||
| Total Costs Excluding Land | $217.06 | $5.43 | ||||
| **** Your Profit Goal **** | $__________ /bu. | |||||
| $$– Price Needed for Profit – $$ | $__________ /bu. | |||||
| *May need to add post herbicide application for grass control in some fields. UGA Extension Agricultural and Applied Economics, 3/2007 |
||||||
| Sensitivity Analysis of CANOLA, DRYLAND | |||||
| NET RETURNS ABOVE VARIABLE COSTS PER ACRE Varying Prices and Yield (Bushels) | |||||
| -25% 30 |
-10% 36 |
Average 40 |
+10 44 |
+25% 50 |
|
| $ 6.00 | $ 18.72 | $ 54.72 | $ 78.72 | $ 102.72 | $ 138.72 |
| $ 6.50 | $ 33.72 | $ 72.72 | $ 98.72 | $ 124.72 | $ 163.72 |
| $ 7.00 | $ 48.72 | $ 90.72 | $ 118.72 | $ 146.72 | $ 188.72 |
| $ 7.50 | $ 63.72 | $ 108.72 | $ 138.72 | $ 168.72 | $ 213.72 |
| $ 8.00 | $ 78.72 | $ 126.72 | $ 158.72 | $ 190.72 | $ 238.72 |
| Estimated Labor and Machinery Costs per Acre | ||||||
| Preharvest Operations | ||||||
| Operation | Acres/ Hour |
No. Times Over | Labor Use (Hr.) | Fuel Use (Gal./Ac) | Machinery Repairs ($/Ac) |
Fixed Costs ($/Ac) |
| Heavy disk 27' with tractor (180-199 hp - MFWD 190 | 13.214 | 1.00 | 0.08 | 0.74 | $ 1.41 | $ 4.08 |
| Chisel plow (folding) 24' with tractor (180-199 hp) - MFWD 190 | 12.982 | 1.00 | 0.08 | 0.75 | $ 1.28 | $ 33.45 |
| Disk & incorporate 32' with tractor (180-199 hp) - MFWD 190 | 15.515 | 1.00 | 0.06 | 0.63 | $ 1.28 | $ 3.38 |
| Grain drill 20' with tractor (180-199 hp) - MFWD 190 | 10.606 | 1.00 | 0.09 | 0.92 | $ 1.93 | $ 5.38 |
| Spray (band) 60' with tractor (120-139 hp) - 2WD 130 | 34.455 | 1.00 | 0.03 | 0.19 | $ 0.24 | $ 0.59 |
| Total Preharvest Fuel, Repairs, Fixed Costs and Labor | 0.340 | 3.23 | $ 6.14 | $ 16.88 | ||
| Harvest Operations | ||||||
| Operation | Acres/ Hour |
No. Times Over | Labor Use (Hr.) | Fuel Use (Gal./Ac) | Machinery Repairs ($/Ac) |
Fixed Costs ($/Ac) |
| Header wheat/Sorghum 22' Rigid with combine (200-249 hp) 240 hp | 6.439 | 1.000 | 0.155 | 1.92 | $ 3.36 | $ 20.44 |
| Corn grain cart 8R36500 bu with tractor (120-139 hp) - 2WD 130 | 10.642 | 1.000 | 0.094 | 0.63 | $ 0.56 | $ 2.34 |
| Total Harvest Fuel, Repairs, Fixed Costs and Labor | 0.249 | 2.55 | $ 3.92 | $ 22.78 | ||
| Prepared by Nathan B. Smith, John Woodruff and David Buntin, UGA Extension Economist, Agronomist and Entomologist. UGA Extension Agricultural and Applied Economics, 3/2007 | ||||||
Summary
In general, productive, well-drained row crop land is needed for canola. Soils that have significant amounts of clay in the B horizon (subsoil layer) are good for canola production provided that tillage provides for deep canola rooting to the subsoil layer. Canola generally does not perform well on poorly drained soils or on deep sands.
Prime Canola Management Strategies
- Contact your local elevator to determine if local buying points are available. (Do this before planning for canola production.)
- Lime land to pH 6.0.
- Deep till land to subsoil layer.
- Disc or field cultivate and apply 1 pt/acre of trifluralin herbicide ahead of planting.
- Plant canola about one month before first expected killing frost.
- Plant seed shallow 1/4 to 1/2 inch deep in 6-8 inch wide rows with a precision seeder.
- Fertilize according to soil test.
- Apply 50 lbs N/A or 2 tons of poultry litter per acre at planting.
- Apply early winter N, 25 to 30 lbs/A if plants become stunted and reddish/purple.
- Apply balance of N in mid-February so that total N/A is about 160 lbs.
- Apply 1 lb of sulfur for every 5 lbs of N applied.
- Scout biweekly for aphids. Treat if 15 percent or more of stand becomes infested.
- Direct harvest canola within 10 days after maturity.
Field Situations Where Conventional Canola Should Not Be Grown
- Deep sands
- Poorly drained soils
- Any soil treated with Cadre or Strongarm herbicide in the past 36 months
- Any soil planted to winter legumes, canola or other Brassicas in the last 24 months
- Fields heavily infested with wild radish
Status and Revision History
Published on Aug 10, 2007
Published on Oct 15, 2010
Published with Minor Revisions on Oct 31, 2013
Published with Full Review on Aug 04, 2016
Published with Full Review on Apr 19, 2017



























































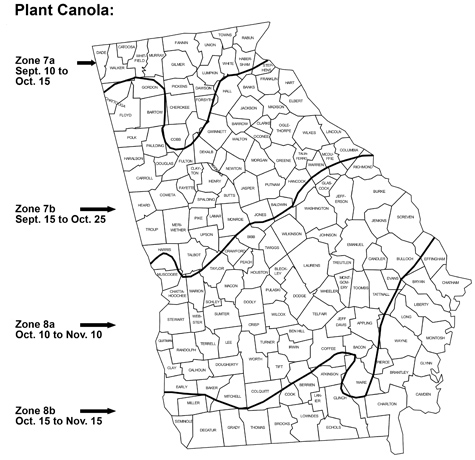 Figure 3. Recommended planting dates based on Plant Hardiness Zones of Georgia.
Figure 3. Recommended planting dates based on Plant Hardiness Zones of Georgia.