- Chemigation
- Fertigation
- Acidification to Remove Mineral Deposits
- Chlorination to Control Algae and Bacteria
Drip irrigation is an important component of vegetable production systems in Georgia. In 1982, drip irrigation was used on fewer than 3,000 acres of Georgia vegetables. Ten years later, more than 17,000 acres of the state’s vegetables were drip-irrigated. This trend is not unique to Georgia. In a 1993 survey of the southeastern United States, 86 percent of respondents indicated acreage of drip irrigation was increasing in their states, and 97 percent of the drip-irrigated vegetables were grown on plastic (polyethylene) mulched beds. More vegetable growers are using plastic-mulched beds and drip irrigation to enhance yields and quality and, in some instances, to promote earlier maturity. Because drip irrigation has proved to be the best irrigation method for vegetables grown on plastic mulch, it has become an integral part of this production system.
Drip irrigation is more desirable than other irrigation methods for several reasons. Two important advantages are (1) water conservation (drip requires about half as much water over the growing season as sprinkler irrigation), and (2) the potential exists for significantly improving fertilizer management.
Fertigation is the timely application of small amounts of fertilizer through drip tubes directly to the root zone. Compared to conventional ground application, fertigation improves fertilizer efficiency. Subsequently, comparable or better yields and quality can be produced with 20 percent to 50 percent less fertilizer.
Mineral precipitates (often seen as scale deposits), algae and bacteria clog drip emitters. Clogged emitters cause variable water distribution during irrigation and uneven fertilizer application during fertigation. Variable water or fertilizer application hinders uniform crop development, reduces yields and jeopardizes quality. For growers to effectively use drip technology, they must prevent clogging drip emitters.
Chemigation
Chemigation Technology
Chemigation is an inclusive term referring to the application of a chemical into or through an irrigation system. It includes the application of fertilizers, acids, chlorine and pesticides.
Fertigation is specifically the application of fertilizer (plant nutrients) through an irrigation system.
Acidification is the introduction of an acid, such as phosphoric, sulfuric or hydrochloric (muriatic) acid into an irrigation system.
Chlorination is the introduction of chlorine, such as liquid sodium hypochlorite (household bleach) or chlorine gas into an irrigation system.
Because drip emitters are small, they clog easily. An adequate filtration system is necessary to prevent the introduction of soil particles (sand, silt and clay) and water-borne debris into drip tubes. Additional anti-clogging techniques include acidification, which prevents or removes mineral precipitates, and chlorination, which removes and prevents the growth of bacteria and algae.
To effectively fertigate crops, growers must properly maintain drip systems so they apply water and fertilizer uniformly. In addition, growers need to determine (1) which fertilizer formulations are most suitable for injection, (2) the most appropriate analysis for specific crops at specific stages of growth, (3) the amount to apply during a given fertigation event, and (4) the timing and frequency of applications.
Benefits of Chemigation
Uniform Application
Chemigation facilitates the uniform distribution and precision placement of fertilizers and other chemicals.
Timely Application
In most cases, materials can be applied regardless of weather or field conditions.
Reduced Application Costs
In general, cost of application by chemigation is about one-third the cost of conventional application methods.
Improved Management
Timely applications of small but precise amounts of fertilizer directly to the root zone allow growers to effectively manage fertilizer programs. This conserves fertilizer, saves money and optimizes yield and quality.
Reduced Soil Compaction
Chemigation reduces tractor and equipment traffic in fields. This reduces soil compaction.
Reduced Exposure to Chemicals
Chemigation minimizes operator handling, mixing and dispensing of potentially hazardous materials. Also, people and non-target crops are not exposed to inadvertent chemical drifts.
Reduced Environmental Contamination
When used with the recommended safety devices, properly-designed and accurately-calibrated chemigation systems help preserve quality of the environment.
Chemigation can save time, reduce labor requirements, and conserve energy and materials. However, chemigation is beneficial only to the extent that the drip irrigation system is adequately designed, fully functional and properly managed.
In many situations, chemigation is as good or better than conventional application methods. However, conventional application is still preferred or required for some materials. Never inject any material that is not labeled and recommended for the crop and for injection through the system. Always follow label directives.
General Principles of Chemigation: Safety Considerations
The irrigation pumping plant and the chemical injection pump should be interlocked so, if the irrigation pumping plant were to stop, the chemical injection pump will also stop. This will prevent chemicals from the supply tank from filling irrigation lines should the irrigation pump stop. With internal combustion engines, the chemical injection pump can be belted to the drive shaft or an accessory engine pulley. Injection pumps driven by electric motors require a separate one-third or one-half horsepower electric motor for the chemical injection pump. Controls for the motors should be electrically interlocked to stop the injection pump motor whenever the irrigation pump stops. This is shown in Figure 1 for electric motors.
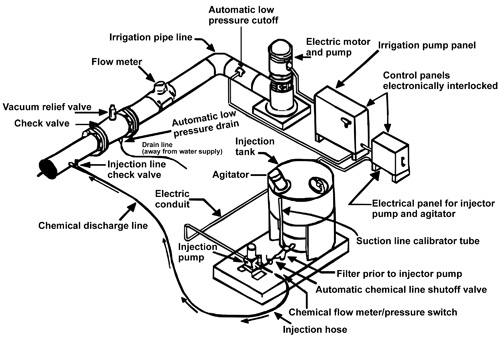 Figure 1. A typical electrically driven chemigation system.
Figure 1. A typical electrically driven chemigation system.Check and vacuum relief valves (anti-siphon devices) are necessary safety devices. They prevent water or mixtures of water and chemicals from draining or siphoning back into the water source. Both valves must be located between the irrigation pump discharge and the point where chemicals are injected into the irrigation pipeline (Figure 1).
A check valve should be installed in the chemical injection line to prevent the back flow of water from the irrigation system into the chemical supply tank. If the injection pump stops and there is no check valve, irrigation water can flow through the injection line into the chemical supply tank. Subsequently, the tank may overflow and cause a chemical spill around the water source. Chemicals from such spills can contaminate ground and surface water.
An additional safety item is a small, normally closed solenoid valve to be electrically interlocked with the engine or motor that drives the injection pump. This solenoid valve provides a positive shutoff in the chemical injection line, which stops chemical or water flow in either direction if the injector pump stops.
For automated control, a pressure switch should be electrically interlocked with the safety panel on the irrigation system. This switch will automatically shut down the irrigation system and the injection pump if pressure is lost in the injection discharge line. Usually, loss of pressure in injection lines occurs when the chemical tank is pumped dry.
The American Society of Agricultural Engineers (ASAE) standard EP 409.1 can be used as a general guideline for backflow prevention devices. However, if you are chemigating in Georgia, keep in mind that the Georgia Department of Agriculture enforces backflow prevention regulations in Georgia. Please contact the Georgia Department of Agriculture for current guidelines.
Injection Pumps
Two basic types of injection pumps — the Venturi (Figure 2) and the metering pump (Figure 3) — are commonly used for injecting fertilizer and other chemicals into drip-irrigation systems. Field setups for both types should have an adjustable injection rate. Any components that will be in contact with fertilizer, chlorine or acid should be resistant to corrosion.
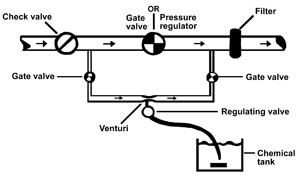 Figure 2. Venturi chemical injector.
Figure 2. Venturi chemical injector.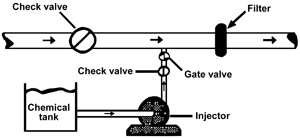 Figure 3. Chemical metering pump.
Figure 3. Chemical metering pump.
Venturi
The Venturi system creates a pressure differential that forms a vacuum. As water flows through the tapered Venturi orifice, a rapid change in velocity occurs. This velocity change creates a reduced pressure (vacuum), which draws (pulls) the liquid to be injected into the system. Since the injection rate will vary with the pressure differential across the Venturi, a precise regulating valve and a flow meter are recommended for calibrating the system.
Metering Pump
Positive displacement metering pumps are often used to inject chemical solutions into drip irrigation systems. Portable positive displacement pumps can be moved from field to field. Metering pumps may be powered by small electric motors or by hydraulic drive systems. Hydraulic drive systems use the water pressure in the system to power the pump. In the past, injection rates of positive displacement pumps were adjusted by changing the length of the piston stroke. However, injection rates of some of the more recent models can be adjusted with a variable frequency drive. This drive varies the speed of the injection pump with the flow rate of the irrigation system.
Diluting Chemical to Be Injected
Injection pumps must be accurately calibrated by properly adjusting the injection rate. Ideally, an injection pump should be capable of being adjusted to the desired injection rate. However, it is not always possible to obtain an injector pump that accurately injects at low chemical injection rates (commonly encountered with small drip systems). If the injector is to also inject fertilizer, it will need sufficient capacity for injecting fertilizer. Injection rates for fertilizers are usually much higher than injection rates for chemicals such as liquid chlorine or acid.
In some situations, it may not be possible to lower the injection rate enough to inject concentrated solutions at the desired rate. This problem can usually be overcome by adding a precise amount of water to dilute the concentration of the active ingredient in the solution (see example under “Chlorination to Control Algae and Bacteria”).
Point of Injection
Chemicals should be injected into the system at a point before the filters. Filters help prevent particulate
matter which may be in the chemical solution from entering the irrigation system and causing clogging problems.
Determining Injection Rate
Before calibrating the injection pump, determine the desired injection rate. Use the following steps as a guide.
- Determine the area (acres) to be chemigated.
- Determine the volume of chemical solution (gallons) to be applied per acre.
- Determine the total number of gallons needed to treat the area (step 1 x step 2).
- Determine how long (hours) the system will be run during this chemigation event.
- Calculate the desired injection rate in gal/hr (step 3 divided by step 4).
- Use the following equations to convert gallons per hour (gal/hr) to milliliters per minute (ml/min) or ounces per minute (oz/min). Equation 1: 63.09 x gal/hr = ml/min. Equation 2: 2.13 x gal/hr = oz/min.
Example: 10 acres are to be chemigated with 1.3 gal of solution per acre and the chemical is to be injected for 1 hour. (A) How many gallons of chemical solution will be required? (B) What is the desired injection rate? (C) How many ml of solution would a correctly calibrated pump inject each minute?
- Determine how many gallons of chemical solution are required (step 1 x step 2). 10 acres x 1.3 gal per acre = 13 gal. Therefore, 13 gallons of chemical solution are required for 10 acres.
- Determine the injection rate (step 3 divided by step 4). Thirteen gallons divided by 1 hour = 13 gal/hr. Therefore, the desired injection rate is 13 gallons per hour.
- Determine how many ml of solution are to be injected each minute (equation 1). 63.09 x 13 gal/hr = 820.17 ml/min. Therefore, a correctly calibrated injection pump will inject 820 ml (rounded to nearest whole number) of chemical solution in 1 minute.
Calibrating the Injection Pump
Chemigation should never be attempted without accurate calibration. Manufacturers’ suggested settings are helpful guides. However, to ensure that recommended amounts are being applied at the desired concentrations, calibrate the injection pump on-site.
The objective of calibrating the injection pump is to adjust the pump injection rate to the desired injection rate. The pump injection rate is determined by measuring the volume of solution pumped through the injection pump (injected volume) during a specific duration of time (usually 60 to 120 seconds).
The injected volume can be determined by any of the following methods:
Method 1 — Using a graduated cylinder, measure a selected volume of the solution to be injected. The selected volume should be of sufficient quantity to allow injection for several minutes. Place this known volume into a container connected to the intake line of the injection pump. With the system operating and fully charged, activate the injection pump and determine the number of seconds required for this known volume to be injected.
Method 2 — This method is similar to the above method. The primary difference is, in method 2, only a portion of the measured chemical solution is injected. Using a graduated cylinder, measure a selected volume of the solution to be injected. This selected volume should be of sufficient quantity to allow injection for several minutes. Place this known volume into a container connected to the intake line of the injection pump. With the system operating and fully charged, activate the injection pump for a specific duration of time. This injection period should be for several minutes. However, it should be short enough so that only a portion of the solution is injected. At the completion of the injection period, measure the volume of solution left in the container. The volume of injected solution is determined by subtracting the amount remaining after injection from the original volume.
Method 3 — In this method, the solution pumped through the injection pump during a given period of time is collected and measured. With the system operating and fully charged, activate the injection pump for a specific time (2 to 5 minutes). Divert the output line from the injection pump into a container. A pressure regulating device should be installed on the output line to simulate system back pressure. Measure the output with a graduated cylinder to determine the volume of chemical injected.
Since operating pressures and flow characteristics of irrigation systems may influence injection rates, it is necessary to perform calibration procedures with the irrigation system operating and fully charged. Before beginning calibration, make sure the system is primed, that it is operating at the same pressure it will be during injection, and that suction and discharge lines do not contain air bubbles. Also, during calibration, keep the point of injection at the same height that it will be during actual chemigation. Once the pump’s injection rate has been determined, this rate can be adjusted until the desired injection rate is achieved.
Example — Using the calculations from the previous example and following manufacturer’s operating instructions, set the injector pump to 13 gallons per hour (from step 5 of the previous section, “Determining Injection Rate”). With the system running, fully charged and the point of injection at the same height that it will be during actual injection, collect and measure the solution pumped through the injector pump in 60 seconds. The volume should be 820 ml (from step 6). If any amount other than 820 ml is pumped through the injector pump, re-adjust the injector pump setting and repeat this procedure until 820 ml are collected in 60 seconds. When 820 ml are collected in 60 seconds, the injector pump is calibrated to inject at the desired rate of 13 gallons per hour.
Fertigation
Fertilizers are the chemicals most often injected int drip irrigation systems. The potential for fertigation is one of the primary reasons many growers install drip irrigation. Properly managed applications of plant nutrients through drip systems significantly enhance fertilizer efficiency while maintaining or increasing yield. On the other hand, poorly-managed fertigation may result in substantial yield losses.
Fertilizers are available in different forms and concentrations. Formulations usually contain two or more nutrients and the solubilities of various formulations vary significantly. Fertigation involves deciding which nutrients (and how much) to apply, selected the most effective formulations, properly preparing solutions for injection and scheduling injections (Table 1) to ensure that essential nutrients are available as needed.
| Crop | Estab. Methodw |
Typical Bed Spacing (ft) |
Total N |
lb/A* K2O |
Crop Development | Injection Rate (lb/A/day) N and K2O |
|
|---|---|---|---|---|---|---|---|
| Stage | Weeksyz | ||||||
| Cantaloupe (Muskmelon) |
TP | 5 | 120 | 120 | 1 | 2 | 1.0 |
| 2 | 3 | 1.5 | |||||
| 3 | 3 | 2.0 | |||||
| 4 | 2 | 1.5 | |||||
| 5 | 2 | 1.0 | |||||
| Collard | TP | 6 | 120 | 120 | 1 | 3 | 1.5 |
| 2 | 6 | 2.0 | |||||
| Cucumber | S | 5 | 120 | 120 | 1 | 1 | 1.0 |
| 2 | 2 | 1.5 | |||||
| 3 | 6 | 2.0 | |||||
| 4 | 1 | 1.5 | |||||
| Eggplant | TP | 6 | 120 | 120 | 1 | 2 | 1.0 |
| 2 | 2 | 1.5 | |||||
| 3 | 6 | 2.0 | |||||
| 4 | 3 | 1.5 | |||||
| Pepper | TP | 6 | 160 | 160 | 1 | 2 | 1.0 |
| 2 | 3 | 1.5 | |||||
| 3 | 7 | 2.0 | |||||
| 4 | 1 | 1.5 | |||||
| 5 | 1 | 1.0 | |||||
| Pumpkin | S | 8 | 120 | 120 | 1 | 2 | 1.0 |
| 2 | 2 | 1.5 | |||||
| 3 | 4 | 2.0 | |||||
| 4 | 2 | 1.5 | |||||
| 5 | 1 | 1.0 | |||||
| Tomato | TP | 6 | 160 | 160 | 1 | 2 | 1.0 |
| 2 | 3 | 1.5 | |||||
| 3 | 7 | 2.0 | |||||
| 4 | 1 | 1.5 | |||||
| 5 | 1 | 1.0 | |||||
| Summer Squash | S | 5 | 120 | 120 | 1 | 2 | 1.0 |
| 2 | 2 | 1.5 | |||||
| 3 | 2 | 2.0 | |||||
| 4 | 5 | 1.5 | |||||
| 5 | 1 | 1.0 | |||||
| Watermelon | S | 8 | 120 | 120 | 1 | 4 | 1.0 |
| 2 | 2 | 1.5 | |||||
| 3 | 2 | 2.0 | |||||
| 4 | 3 | 1.5 | |||||
| 5 | 2 | 1.0 | |||||
| Winter Squash | S | 8 | 120 | 120 | 1 | 3 | 1.0 |
| 2 | 3 | 1.5 | |||||
| 3 | 2 | 2.0 | |||||
| 4 | 4 | 1.5 | |||||
| 5 | 1 | 1.0 | |||||
| z Includes any starter fertilizer. y Where 20% of N and K2O have been applied as starter, injections can be omitted for 1 or 2 weeks. x For extended-season crops, N maintenance applications can proceed at 1.0 to 1.5 lbs/A/day. Use tissue testing to fine-tune amounts. w Establishment method (seed or transplant) affects the schedule. Transplanting shortens growth cycle and injection schedule by 1-2 weeks. Note. This table is adapted from "Fertilizer Application and Management for Micro (or Drip) Irrigated Vegetables in Florida." Florida Cooperative Extension Special Series Report SS-VEC 45, April 1991, co-authored by George J. Hochmuth and Gary A. Clark. Used with permission. |
|||||||
This section specifically addresses types of fertilizer formulations, fertigation strategy, and fertilizer injection rates. For a more complete discussion on effectively managing fertigation, see University of Georgia Extension Bulletin 1108, Plasticulture for Commercial Vegetable Production, available from your county Extension office.
Many sources of nitrogen and potassium are suitable for injection through drip irrigation systems. They include various nitrogen solutions, ammonium nitrate, calcium nitrate, potassium nitrate and potassium chloride. Granular fertilizer, liquid fertilizer or a combination of the two may be used when fertigating.
Solubility of Fertilizer Formulations
Solubility indicates the relative degree to which a substance dissolves in water. Solubility of fertilizer is a critical factor when preparing stock solutions for fertigation, especially when preparing fertilizer solutions from dry fertilizers. As indicated in Table 2, fertilizer formulations vary considerably in their ability to dissolve in water.
Hot water increases solubility and makes dissolving fertilizer easier and quicker. Hot water may be especially helpful when dissolving a fertilizer such as potassium nitrate, which actually cools the solution as it dissolves.
Because solubility is reduced when water cools, it is not a good practice to heat water in order to dissolve “extra” fertilizer (more than is soluble at normal temperatures). As the solution cools, this extra fertilizer will come out of solution (precipitate or “salt out”) and possibly clog drip emitters.
Growers routinely make large quantities of fertilizer stock solutions for injection during several fertigations over a period of time. When making stock solutions that will not be injected soon after preparation, keep in mind that solubilities decrease when the solutions are cool. If maximum amounts of fertilizer are dissolved in stock solutions and these solutions are cooled during the night (which occurs frequently in early spring and fall), some of the fertilizer may come out of solution. Therefore, it is generally not advised to dissolve maximum amounts of fertilizer in stock solutions that will be injected at some future time.
Sometimes growers dissolve two or more fertilizer formulations in the same stock solution. Keep in mind that the solubilities listed in Table 2 apply only when fertilizer is dissolved in pure water (water essentially free of minerals and other contaminants). Once fertilizer is dissolved in pure water, the purity of the water and the solubility of additional fertilizers in that solution are affected. The solubilities shown in Table 2 will not apply in such situations.
| Fertilizer Formulation | Solubility (lb/gal) |
|---|---|
| Ammonium nitrate | 9.8 |
| Calcium nitrate | 8.5 |
| Potassium chloride | 2.3 |
| Potassium nitrate | 1.1 |
If two or more fertilizers are to be mixed in the same solution, test their combined solubility by mixing them in one to five gallons of water (mix precise amounts so the concentrations will be the same as the concentrations desired in the stock solution). If the fertilizers dissolve completely in this test, proceed with making the planned stock solution. If the fertilizers do not dissolve, consider making less concentrated solution or possibly using some other fertilizer(s) to make the stock solution.
Liquid Fertilizer Formulations
Preparation of nutrient stock solutions from dry fertilizers may require considerable time and effort and can generate sediments and scums as waste products. Therefore, commercially prepared liquid fertilizer solutions (true solutions, not suspensions) that are completely water soluble are often used. Liquid fertilizers are available in a variety of grades (4-0-8, 7-0-7, etc.) and can be purchased with or without micronutrients. A liquid formulation of calcium nitrate (9% N, 11% Ca) is also available. Liquid formulations such as these are very convenient, because they can be injected directly (without mixing in water) with a variable rate injection pump.
Although transportation costs make liquid formulations a little more expensive, they save time and labor and help prevent problems associated with poorly made “home mixes.” Also, they eliminate the problems caused by insoluble materials found in some dry fertilizers.
Even with liquid formulations, be careful when injecting fertilizers containing phosphorus or sulfur into drip systems. Phosphorus and sulfur may react with calcium and/or magnesium in the irrigation water to form mineral precipitates that could clog emitters. Micronutrients can also cause problems due to precipitation. If micronutrients must be injected, use soluble chelated forms that are less likely to precipitate. If you are unsure about formulations containing micronutrients, sulfur or phosphorus, consult your supplier or test them prior to injections.
Granular Fertilizer Formulations
Completely soluble granular fertilizers are readily dissolved in stock solutions. However, there are some granular formulations that are not completely water soluble (all the fertilizer does not dissolve). Because they do not completely dissolve in stock solutions, these formulations often cause serious clogging problems in drip systems. Undissolved fertilizer can quickly clog drip emitters and result in variable (nonuniform) application of water and nutrients. Granular fertilizers should be completely dissolved in the nutrient stock solution before injection is attempted.
Compared to liquid fertilizer, more effort may be required to dissolve granular fertilizer formulations. For best results, start by putting about half the required amount of water into the tank. Then, while continuously stirring/agitating the water, begin adding fertilizer (in small increments) until the desired quantity of fertilizer is dissolved in the stock solution. If the fertilizer does not readily dissolve, add more water while mixing. Be careful that you don't add too much water. After the fertilizer is dissolved, add any additional water needed to bring the volume to the required amount.
If you are unable to dissolve the desired amount of fertilizer in the desired volume of water, the solubility of the selected fertilizer is too low for the concentration you have chosen. If solubility is a problem, consider (1) making a less concentrated (more dilute) solution and increasing the duration of injection or (2) using a more soluble fertilizer formulation.
Dry fertilizers containing non-dissolving filler or coating materials are especially troublesome. However, if any of these types of fertilizer are used, allow a settling period (6 to 8 hours) before injection, so sediments can settle to the bottom of the tank. Prior to injection, adjust the injection pump intake so it is 8 to 10 inches above the surface of the sediments. This prevents injection of these sediments and subsequent clogging of lines and emitters. A flush valve should be located at the bottom of the tank to facilitate removal of sediments after injection of the stock solution.
Also, surface scums may form when dissolving fertilizers that contain fillers or coatings. When this occurs, the scum should be skimmed off or drawn off through a port located near the top of the stock solution. Sediments and surface scums are chemical waste pro-ducts that must be handled and disposed of according to state and federal regulations. It is best to use fertilizers that are specifically formulated for dissolving in water when fertigating with drip systems.
Formulating Stock Fertilizer Solutions
The following steps can be used to formulate stock solutions for injection.
Step 1. Convert the fertilizer from pounds of formulated material to pounds of actual plant nutrients (active ingredients), such as nitrogen (N) and potash (K2O).
Step 2. Calculate the total pounds of active ingredient (a.i.) to be dissolved in the stock solution by multiplying the pounds of active ingredient to be applied per acre by the number of acres to be fertigated.
Step 3. Convert the total pounds of a.i. to pounds of fertilizer (as formulated) to determine the amount of fertilizer to put into the mixing tank.
Dissolve the required weight of fertilizer determined in step 3 in water (80 percent to 90 percent of the volume to be injected) and then add precisely enough water to bring the volume of the stock solution up to the required amount.
Fertilizer Injection
Factors crucial to effective fertigation include (1) nutrient concentrations of fertilizer solutions, (2) the amount of fertilizer to be injected during each fertigation, (3) the total amount of fertilizer to be injected during the production season, (4) the durations of injection periods, and (5) the frequency (scheduling) of fertigations.
Nutrient concentrations in fertilizer stock solutions can range from very weak to near maximum strength. The maximum strength (highest possible concentration) of a stock solution is limited by the solubility of the fertilizer(s) dissolved in the solution.
The amount of nutrients to be applied during any given fertigation and the total amount to be applied during the production season depend on the frequency of fertigation, soil type and nutrient requirements of the crop. These topics are discussed in detail in University of Georgia Cooperative Extension Bulletin 1108, Plasticulture for Commercial Vegetable Production.
Injection Rate
Injection rate refers to the volume of solution injected during a specific duration of time. The injection rate (usually stated in gallons per hour) is determined by dividing the volume (gallons) to be injected by the duration of injection (hours).
Injection Duration
A minimum injection time of 45 to 60 minutes is recommended. This time is sufficient for uniform distribution of nutrients throughout the fertigation zone. Injection of “slugs” (highly concentrated solutions of fertilizer usually injected in much less than 45 minutes) is not recommended. They often result in nonuniform fertilizer applications and subsequent poor crop performance.
Limit injection time to prevent the application of too much water, because excessive water leaches plant nutrients below the root zone. In addition, too much water saturates the soil, causing damage to roots and plants.
Also, extended injection times may make it difficult to schedule irrigation as needed to all zones. Drip irrigation systems are normally set up in land area zones such that only one zone can be fertigated/irrigated at the time. The time available for irrigating all zones combined cannot exceed 24 hours per day. On extremely hot days, vegetable crops often need to be irrigated at least once, sometimes twice every 24 hours. In these situations, extended injection periods can take up so much time that 24 hours elapse before all zones are irrigated sufficiently.
The maximum injection time depends on soil type and nutrient and water requirements of the crop. However, as a general rule, a “reasonable” maximum duration of injection should not exceed 2 hours per zone.
Acidification to Remove Mineral Deposits
Acid Injection
Mineral precipitates can form deposits (scale) that clog emitters. The most common deposits are calcium or magnesium carbonates and iron oxides. Since precipitation occurs more readily in water with a high pH (above 7.0), precipitation of these compounds can be prevented by continuous injection (whenever the system is operating) of a small amount of acid to maintain water pH just below 7.0.
A more popular control method is to remove deposits as they are formed by periodic injection of a greater volume to acid. Enough acid should be injected continuously for 45 to 60 minutes to reduce the water pH to 4.0 or 5.0.
Phosphoric acid (which also supplies phosphate to the root zone), sulfuric acid, or hydrochloric (muriatic) acids are commonly used. The selection of a specific acid depends on cost and availability, water quality, the severity of clogging, and nutrient needs of the crop.
The amount of acid required to treat a system depends on (1) the strength of the acid being used, (2) the buffering capacity of the irrigation water and (3) the pH (of the irrigation water) needed to dissolve mineral precipitates in lines and emitters. The required pH of the irrigation water (target pH) depends on the severity of mineral deposits. Experience is helpful when estimating target pH.
To determine the volume of a selected acid needed at a specific site, estimate the target pH and run a “titration” test (as described below) using the selected acid and irrigation water from the site. This test will indicate the volume of acid required to lower the pH of a selected volume of water to the target pH. Titration provides an acid volume:water volume ratio that can be used in conjunction with the system flow rate to deter-mine the appropriate acid injection rate. The acid injection rate is determined by dividing the volume of water by the flow rate of the irrigation system and multiplying the result by the volume of acid added to reach the target pH.
Titration
A water container, a non-corrosive measuring cup, beaker or pipette calibrated in small increments such as milliliters, and a portable pH meter are needed to run the titration test. The volume of the container may be as small as 10 liters (about 3 gallons) or as large as 55 gallons. In general, the smaller the increments used when measuring and dispensing the acid into water, the smaller the required container.
To run the titration test, put a known volume of water (from the site) into the container and check the pH. Add a small amount of acid (1-3 ml for 3 gallons, 4-8 ml for 30 or more gallons) to the water, stir and re-check the pH. Continue this process until the target pH is attained. As the acidity of the water nears the target pH, add acid in very small increments (1 ml); otherwise, the pH may quickly drop below the target pH and necessitate repeating the test. Always add acid to water. Caution: Never add water to acid.
The following example illustrates how to determine the required volume of acid and the appropriate acid injection rate.
Example: For a system with a flowrate of 200 gal/min.
- Based on the severity of mineral deposits in the system, a target pH of 4.5 and an injection period of one hour are selected.
- Put 50 gallons of water into a 55-gal drum. Check the pH. Meter indicates pH of 7.4.
- Add 8 ml phosphoric acid. Check the pH. Meter indicates pH of 6.9.
- Add 7 more ml phosphoric acid. Check the pH. Meter indicates pH of 6.0.
- Add 4 more ml phosphoric acid. Check the pH. Meter indicates pH of 5.3.
- Add 1 more ml phosphoric acid. Check the pH. Meter indicates target pH of 4.5.
- 20 ml (8+7+4+1) of phosphoric acid were required to lower the pH of 50 gal of water to the target pH of 4.5.
- Divide 50 gal by the system flowrate of 200 gal/ minute and multiply the result by the ml of phosphoric acid required to reach the target pH. 200 gal divided by 50 gal = 4. 4 x 20 ml = 80 ml phosphoric acid. Therefore, the required acid injection rate is 80 ml per minute.
- Multiply 80 ml per minute by the injection time to determine the required volume of acid needed during the 1 hour injection period. 80 ml x 60 min = 4,800 ml (approximately 1.3 gal, since there are 3785 ml in 1 gallon)
Note: Acid injection rates are usually very low (ml/ hour). Although injection pumps with low flow rates may be suitable for acid injection, they may not have enough capacity for injecting fertilizers.
After the desired amount of acid has been injected and distributed throughout the irrigation system, turn the system off and let the low pH water remain in the lines for several hours, preferably overnight. This allows sufficient reaction time for the acidified water to dissolve mineral precipitates. After the setting period, flush the lines to remove dislodged and solubilized materials. To flush the lines, bring the system to full charge by running the irrigation pump (injection pump off) until the system reaches normal operating pressure. With the irrigation pump running, begin sequentially opening the ends of the PVC lines and emitter lines to flush the system. To ensure proper flushing, do not open so many lines at one time that system pressure drops below normal levels. If too many lines are opened at one time, the pressure drops too low and the system will not flush adequately. Improperly flushed lines after acidification will likely result in severe clogging problems. Keep in mind that routinely flushing lines with non-acidified irrigation water will also help remove mineral precipitates from the system.
Chlorination to Control Algae and Bacteria
Algae and Bacteria
Algae — Fresh water algae are microscopic green plants that require light for growth. When we see algae in ponds, we are actually looking at colonies of algae. If algae get inside irrigation lines, they reduce water flow through pipes and eventually clog emitters. Because algae require light for growth, they do not grow in buried pipelines, in black polyethylene laterals or in other conduits that effectively prevent light penetration.
Although enough light may enter exposed white PVC pipes or fittings to permit algal growth, this problem can be prevented by painting PVC pipes and fittings with a blue gloss paint. If algae are growing in your irrigation pond, apply chemical treatment according to the recommendations in the current Georgia Pest Management Handbook. Treatment of the water source and adequate filtration will prevent the introduction of algae into your chemigation system.
Bacteria — Some bacteria can live inside pipelines and drip tubes. They form bacterial slime, which clogs emitters. Bacterial clogging problems in drip irrigation systems are usually caused by sulphur and iron bacteria. These bacteria and the nutrients required for their growth can be present in both well and surface water.
Chlorine Injection Interval
Chlorine injection will prevent clogging of lines and emitters by algae and bacterial slime. Continuous injection of small amounts of chlorine maintains low concentrations of chlorine in the system and prevents their growth. However, periodic injection of larger amounts of chlorine is the preferred treatment for controlling algae and bacteria in drip systems.
You do not need to inject chlorine if you are using municipal water that is already chlorinated. However, if your irrigation water has not been chlorinated, you should be prepared to inject chlorine as needed. Vegetables are often sequentially cropped with the same drip system. In these situations, it is advisable to chlorinate the system at the end of each cropping season or more often if bacterial clogging occurs. If water quality is extremely poor, it may be necessary to chlorinate at the end of each irrigation cycle. Experience is helpful when determining the appropriate intervals between chlorine injections.
Recommended Chlorine Formulations
Liquid sodium hypochlorite (NaOCI) is the easiest form of chlorine to handle and is the type most often used for treatment of drip irrigation systems. It is readily available in supermarkets and other stores as common household bleach (5.25% chlorine). Liquid chlorine is also available from some swimming pool companies as a 10 percent chlorine solution.
Chlorine gas (Cl2) can be injected. Although it is an inexpensive source of chlorine, chlorine gas is more difficult to handle and requires more expensive injection equipment. In addition, chlorine gas is very poisonous and must be handled with extreme caution.
Caution: Powdered calcium hypochlorite Ca(OCl2), also called High Test Hypochlorite (H.T.H.) is a dry powder commonly used in swimming pools. However, H.T.H. is not recommended for injection into drip irrigation systems. When mixed with water (especially at high pH), the calcium contained in H.T.H. can form precipitates.
Initial Chlorine Injection Rate
As chlorine is injected, some of it reacts with bacteria (as it destroys the bacteria) and other forms of organic matter in the irrigation lines. This “reacted” chlorine is chemically bound or “tied up” and is no longer antibacterial. Chlorine that has not reacted re-mains as “free residual chlorine.” Only this free chlorine is available to destroy bacteria and to continue treatment of the system.
For chlorination to be effective, you should maintain 1 to 2 ppm free chlorine in the system for 30 to 60 minutes. Usually, an initial concentration of 5 to 6 ppm is required in order to maintain 1 to 2 ppm free chlorine. Samples for determining the initial chlorine concentration should be taken near the point of injection. Samples should be taken far enough past the point of injection that the chlorine is uniformly mixed in the irrigation water.
The following equation can be used to calculate the injection rate.
Injection rate gal/hr = 0.03 x GPM divided by % chlorine
Example: The desired initial chlorine concentration in irrigation water just past the point of injection is 5 ppm. Assume a drip irrigation system with a total flow-rate of 100 gallons per minute (gpm) and that common chlorine bleach (5.25% chlorine) will be injected.
Injection rate = 0.03 x GPM divided by % chlorine
= 0.03 x 100 divided by 5.25
= 0.57 gal/hr
The chlorine solution must be in contact with algae and bacteria for at least 30 minutes to successfully treat the drip irrigation system. To ensure that all parts of the system receive a minimum of 30 minutes’ contact time, inject chlorine for 1 hour.
For convenience, the injection rates (gal/hr and oz/hr) required to give an initial concentration of 5 ppm chlorine have been calculated for selected flow rates in Table 3.
| Water Flow (gpm) | 5.25% Chlorine Solution | 10% Chlorine Solution | ||
|---|---|---|---|---|
| gal/hr | oz/hr | gal/hr | oz/hr | |
| 10 | 0.06 | 7.7 | 0.03 | 3.8 |
| 20 | 0.11 | 14.1 | 0.06 | 7.7 |
| 30 | 0.17 | 21.8 | 0.09 | 11.5 |
| 40 | 0.23 | 29.4 | 0.12 | 15.4 |
| 50 | 0.29 | 37.1 | 0.15 | 19.2 |
| 75 | 0.43 | 55.0 | 0.22 | 28.2 |
| 100 | 0.57 | 73.0 | 0.30 | 38.4 |
| 150 | 0.86 | 110.0 | 0.45 | 57.6 |
| 200 | 1.14 | 145.9 | 0.60 | 76.8 |
| 250 | 1.43 | 183.0 | 0.75 | 96.0 |
| 300 | 1.71 | 218.9 | 0.90 | 115.2 |
| 350 | 2.00 | 256.0 | 1.05 | 134.4 |
| 400 | 2.29 | 293.0 | 1.20 | 153.6 |
| 450 | 2.57 | 329.0 | 1.35 | 172.8 |
| 500 | 2.86 | 366.0 | 1.50 | 192.0 |
| * During chlorination, the injection rate should be adjusted to maintain 1 to 2 ppm free chlorine at the emitter farthest from the point of injection. | ||||
Maintaining Free Residual Chlorine Concentration
During chlorination, maintain 1 to 2 ppm free chlorine at the point in the system where the concentration is lowest (usually at the point farthest from injection). If the irrigation water has a pH of 7.5 or less, 1 ppm free chlorine is sufficient. However, for alkaline water with a pH above 7.5, maintain 2 ppm. The free chlorine concentration drops as the chlorine reacts with organic matter in the lines. Therefore, to maintain 1 to 2 ppm free chlorine in the lines farthest from injection, it is often necessary to maintain a concentration of 5 to 6 ppm free chlorine near the point of injection. The specific concentration necessary (near the point of injection in a given zone) depends on water quality and the quantity of bacteria, algae and other organic matter in the lines. Maintain the recommended free chlorine concentration at the most distant emitter for 60 minutes. This requires frequent testing of the free chlorine concentration and subsequent adjusting of the chlorine injection rate if needed.
To ensure the free chlorine concentration is maintained at 1 to 2 ppm, measure free chlorine concentration at the emitter most distant from the injection point approximately 10 minutes after injection is initiated. This can be done by using a D.P.D. (N,N Diethyl-P-Phenylenediamine) test kit, which measures only free residual chlorine. These test kits are available from chemical suppliers and most drip irrigation dealers.
Caution: The orthotolidine type test kit, often used for swimming pools, measures total chlorine content (not free residual chlorine) and, therefore, cannot be used satisfactorily for drip systems.
In cases where the injection pump cannot be calibrated low enough to inject 5.25 percent or 10 percent liquid chlorine at the desired rate, dilute the chlorine solution prior to injection. This permits the use of a higher injection rate within the capacity of the injector pump.
Example: Assume you need to inject gallon of 5.25 percent chlorine into your drip system during a one-hour injection period. If your injection pump can inject no less than 2 gallons per hour, add 1 gallon of water to the 5.25 percent chlorine to give a total chlorine solution of 2 gallons. Then set the injector pump to inject 2 gallons per hour.
Status and Revision History
Published on Feb 01, 2005
Published on Feb 27, 2009
Published with Full Review on Feb 15, 2012
Published with Full Review on Jan 30, 2017
Published with Full Review on Jun 14, 2023
