
- Apples and Pears
- Blueberries
- Brambles (Raspberries and Blackberries)
- Bunch Grapes
- Figs
- Muscadines
- Peaches, Nectarines, and Plums
- Strawberries
- References
Introduction
This bulletin is intended to be used as a pictorial diagnostic guide to identify the most common diseases seen on fruits grown in home landscapes, gardens, and/or orchards in Georgia. Refer to the Homeowner Edition of the Georgia Pest Management Handbook for chemical control recommendations.
In terms of plant disease management in home orchards, an integrated pest management (IPM) approach is necessary to manage plant pathogens and ensure production of quality produce. Using clean plant stock, selecting disease-resistant varieties (when available), sanitation, proper cultural care and control, and maintaining healthy plants are all essential components in minimizing home orchard plant diseases. Most fungicides are largely protectant in nature and must be applied before symptoms are seen.
Keeping records or a journal of past plant diseases will be useful in managing future problems in the home orchard. Remember, when applying pesticides, read the chemical label carefully and follow all instructions written on the label. More specifically, take note of the Preharvest Intervals (PHI – interval of time between when the last chemical spray is applied and when the fruit is harvested) for each individual chemical. The PHI will vary depending on the chemical used.
Apples and Pears
Sooty blotch and fly speck

Diagnostic features: Dull black sooty blotches and individual“fly specks”
Pathogen: Multiple organisms that usually occur together as a disease complex, referred to as SBFS (Peltaster fructicola, Geastrumia polystigmatis, and Leptodontium elatius – sooty blotch; Zygophiala jamaicensis – fly speck)
Comments: This disease complex appears late in the summer/early fall. Pruning is important to increase air circulation. Fruit thinning is also important. Diseases favor moderate temperatures and high humidity. These are superficial diseases, and they do not cause rots. Application (rubbing with a cloth) of a bleach solution (1 ounce household bleach per gallon of water) will help to remove these, but subsequent shelf life of apples is reduced.
Bitter rot


Diagnostic features: Concentric rings of acervuli; V-shaped lesions extending to core of fruit
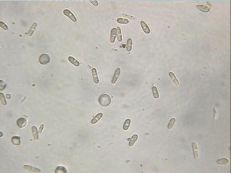
Conidia
(J. Brock, UGA)
Pathogen: Glomerella cingulata
Comments: This is a very important summer disease, especially when conditions are warm and moist! Pustules of spores are formed in concentric rings on the fruit. A sunken, sour-smelling rot results. Good sanitation is vital to management. Remove diseased fruit, which will hang on the tree, and any cankers formed in the woody tissues.
Black rot
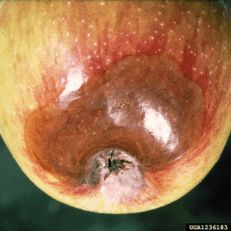
Diagnostic features: Brown, bruised-look on the calyx end of fruit
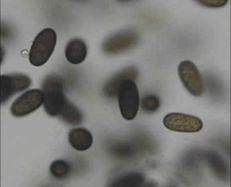
Conidia
Pathogen: Botryosphaeria (Physalospora) obtusa
Comments: A major disease on both apples and pears in the Southeast. On leaves, a symptom known as "frogeye" leaf spot occurs. Infection occurs early in the season at silver tip; rots become evident in the late season at the calyx or bottom end. Rot will be seen as concentric rings, and it will be dark (eventually turning black). Good sanitation is important, so prune out dead wood and remove fallen debris.
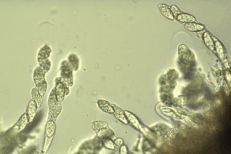
Apple scab
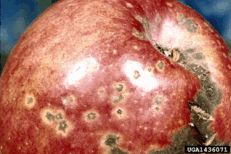

Diagnostic features: Black, scabby lesions on leaves and fruit
Perithecia and spores
Pathogen: Venturia inequalis
Comments: Not a consistent problem in the Southeast. Cool, wet weather favors infection. Fruit and foliage must be protected season-long for adequate management if the disease does occur. Plant resistant varieties (ask local nurseries for availability). Sanitation is important. Rake and destroy fallen leaves to reduce the amount of disease that will carry over to the next year.
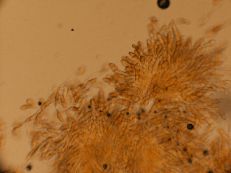
White or bot rot

Diagnostic features: Depressed, soft enlarged lesion on fruit
Ascospores within asci
Pathogen: Botryosphaeria dothidea
Comments: This is a serious and common late-season problem in apples and pears. This fruit rot is a rapidly developing soft rot (unlike bitter rot and black rot, which form harder rots). Sanitation is important. Remove mummified apples (dried, dead apples hanging in the tree) and prune out deadwood.
Fire blight


Diagnostic features: Shepard’s crook symptom on foliage; dieback on branch due to presence of a canker
Pathogen: Erwinia amylovora - Bacterial disease
Comments: This is a bacterial disease, and it is very destructive on both apples and pears. It's difficult and expensive to control. Avoid spraying too often, as resistance may develop. Succulent tissues are most vulnerable to infection, so avoid excessive nitrogen fertilization. Avoid pruning during and after the blossom period (corresponds to insect feeding). Promptly prune out any blighted tissue; remove infected plant parts through cutting 8 to 12 inches below diseased tissue; between cuts, disinfect pruning tools using a 10 percent bleach solution.
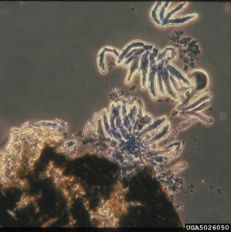
Cedar-apple rust
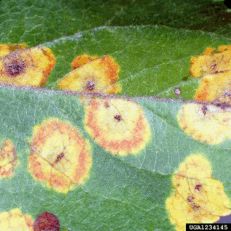

Diagnostic features: Lesions on apple leaves; telial gall on cedar (alternate host)
Teliospores
Pathogen: Gymnosporangium juniperi-virginianae
Comments: Can cause extensive defoliation of apple trees. Plant resistant varieties! If possible, remove galls from nearby cedar trees (breaks the fungal life cycle, as it needs both hosts to reproduce).
Blueberries
Botrytis blight


(Bill Cline, NCSU)
Diagnostic features: Blighted flowers (covered in conidia); berries covered in conidia

Conidiophore and conidia
Comments: Disease affects green twigs, flowers, leaves and fruit. Outbreaks often occur after freeze injury to flowers in the spring, especially when followed by cool, wet weather. Fruit rot does not generally occur until after fruit is harvested. Sanitation is important. Remove infected fruit/mummies and maintain a good mulch layer.
Mummy berry

(H. Scherm, UGA)

(Bill Cline, NCSU)

(H. Scherm, UGA)

(H. Scherm, UGA)

(Bill Cline, NCSU)
Diagnostic features: Shoot blight; mummified berries; apothecia

Conidia
Pathogen: Monilinia vaccinii-corymbosi
Comments: Sanitation is important; rake and remove mummies (dead fruit on the ground); prune annually.
Septoria leaf spot

(Bill Cline, NCSU)
Diagnostic features: Small leaf spots with tan center and purple border (black dot in center – pycnidia of pathogen)

Narrow, filiform several-celled conidia
Pathogen: Septoria albopunctata
Comments: Rake and remove infected leaf debris. Summer pruning or topping will help remove older, infected tissues. Increased spacing will improve air circulation, resulting in dryer foliage.
Twig blight and fruit rot

(Bill Cline, NCSU)

(Bill Cline, NCSU)
Diagnostic features: Dieback of blueberry twigs, rotting berries

Conidia (two types: alpha [oval or fusoid] and beta [long and curved])
Pathogen: Phomopsis vaccinii
Comments: Twig blight – remove infected twigs in winter; choose resistant cultivars when available. Fruit rot – harvest fruit before it becomes overripe.
Brambles (Raspberries and Blackberries)
Anthracnose

(NCSU/PDIC, courtesy Bill Cline)
Diagnostic features: Small, purplish or tan, slightly raised
or sunken spots along young canes
Pathogen: Elsinoe veneta
Comments: Disease affects canes, leaves, fruit and stems of berry clusters. Symptoms on canes are ash grey lesions with raised purple to brown borders. Sanitation is very important. After harvest, cut old floricanes to the ground, and remove and destroy them.
Orange rust

 Orange rust of blackberry, aecial stage (Charles Mims, UGA CAES Department of Plant Pathology)
Orange rust of blackberry, aecial stage (Charles Mims, UGA CAES Department of Plant Pathology) Diagnostic features: Yellow-orange pustules on leaf surfaces (usually lower leaf)
 Orange rust of blackberry, spermogonial stage (Charles Mims, UGA CAES Department of Plant Pathology)
Orange rust of blackberry, spermogonial stage (Charles Mims, UGA CAES Department of Plant Pathology) 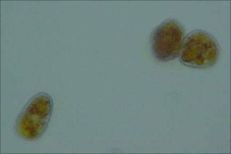
Spores
Pathogen: Kunkelia nitens
Comments: Attacks all brambles except red raspberries. Establishes a systemic infection and, once infected, no cure is available. Symptoms include stunting and limited fruit production. Symptoms occur shortly after leafing out. When disease is first detected, dig up and discard/destroy any infected plants to reduce spread.
Rosette or double blossom
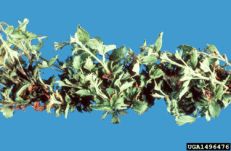
Diagnostic features: Bunchy growth at nodes
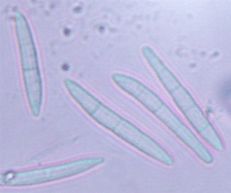
Spores
Pathogen: Cercosporella rubi
Comments: Most damaging to blackberries. In the spring, infected buds from the previous year produce numerous leafy sprouts — "rosettes" or "witches brooms." Berries do not develop from infected blossoms. Remove/destroy nearby wild brambles — they serve as reservoirs; remove infected rosettes and blossom clusters before they open.
Orange felt (orange cane blotch)
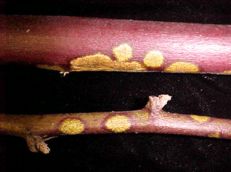
Diagnostic features: Yellow, disc-shaped spots on canes

Algal sporangiophores
Pathogen: Cephaleuros virescens
Comments: Remove old floricanes after harvest; increase air circulation in canopy; avoid stressing plants; improve drainage.
Cane blight

Diagnostic features: Dieback of canes

Pycnidia
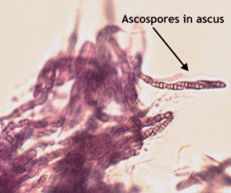
Ascospores in ascus
Pathogen: Leptosphaeria coniothyrium
Comments: Remove old floricanes after harvest; increase air circulation in canopy; avoid stressing plants; improve drainage. Sanitation is very important. Remove dead and infected canes during and after harvest. Avoid stressing plants. During the summer, prune by pinching off tender primocanes when they reach 3-4 feet high. Remove 1-4 inches of primocane tip; avoid making severe pruning cuts on older tissues. Do not prune ahead of predicted rains; prune when 3-4 days of dry conditions are predicted.
Bunch Grapes
Black rot
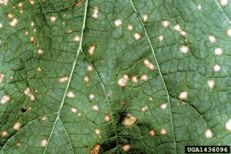


Diagnostic features: Small, yellowish spots on leaves; sunken oval lesion with pycnidia of the fungus (black dots); shriveled mummies (infected berries)
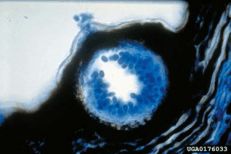
Pycnidia in a mummified grapevine berry
Pathogen: Guignardia bidwellii
Comments: Annual pruning in February; removing infected berries both on the ground and on the plant. After pruning, only the permanent trunk, one-year-old fruiting canes and short spurs should remain. Sanitation is important. Remove mummified fruit! Disease spread is favored by moist, wet weather.
Powdery mildew

Diagnostic features: White powdery fungal growth on berries
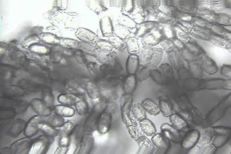
Conidia
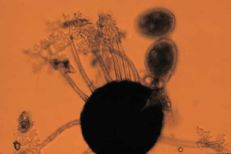
Cleistothecia
Comments: Annual pruning in February will help to remove inoculum; remove infected berries both on the ground and on the plant. After pruning, only the permanent trunk, one-year-old fruiting canes and short spurs should remain.
Downy mildew

Diagnostic features: Yellow, irregular-shaped lesions on upper surface; whitish-gray fungal growth directly under lesions on lower surface of leaves
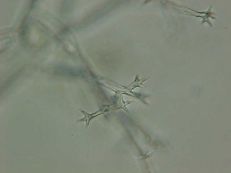
Conidiophores
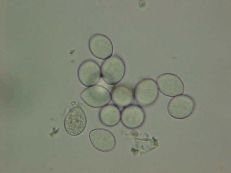
Conidia
Pathogen: Plasmopara viticola
Comments: Annual pruning in February; remove infected berries both on the ground and on the plant. After pruning, only the permanent trunk, one-year-old fruiting canes and short spurs should remain.
Pierce’s disease

Diagnostic features: Scorched leaves with a defined margin and yellow/chlorotic border
Pathogen: Xylella fastidiosa - Bacterial disease
Comments: Vectored by various sharpshooters (such as glassywinged sharpshooter). New growth is stunted, yellow, deformed (resembles zinc deficiency). Choose more resistant cultivars; native grapes are generally more resistant. Do not propagate from symptomatic vines. Do not plant vinifera wine or table grapes at elevations below 1,300 feet. Muscadines are generally resistant, and some other native grapes have limited resistance.
Phomopsis

Diagnostic features: Small, black pycnidia of the fungus on the cane

Two spore types: alpha and beta conidia
Pathogen: Phomopsis viticola
Comments: A late dormant application of lime sulfur is very beneficial for control of this disease.
Botrytis bunch rot

Diagnostic features: Masses of gray conidia covering infected grapes
Pathogen: Botrytis cinerea - see Blueberry botrytis blight
Figs
Root knot nematode
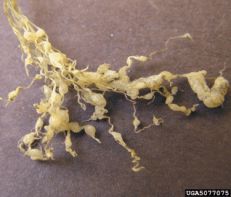
Note: Image not of fig roots (D. Langston, UGA)
Diagnostic features: Knotty, galled roots
Pathogen: Meloidogyne spp.
Comments: Prune tops to balance weakened roots; attentive watering and fertilization may prolong tree life. Nematode infested plants usually die sooner or later regardless of treatment. Plant new trees away from this site!
Rust

Diagnostic features: Small, reddish pustules on the underside of the leaves

Pustule (uredinial stage) on underside of leaf
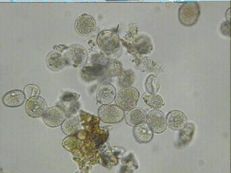
Urediniospores
Pathogen: Cerotelium fici
Comments: Not fatal but will reduce tree vigor and size and quality of fruit. Attacks the leaves, usually in late summer. Infected leaves turn yellow-brown and drop. Underside of leaves have reddish brown spots/pustules. Sanitation is important.
Anthracnose
Pathogen: Glomerella cingulata - see Apple bitter rot
Comments: Not serious; increase air circulation and avoid excess irrigation; sanitation.
Muscadines
Black rot

Diagnostic features: Circular brown leaf spots
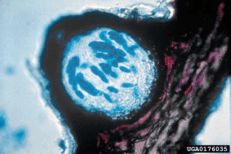
Pycnidia containing conidia
Pathogen: Guignardia bidwellii
Comments: Remove fallen debris and mummified fruit; during the winter, remove all old fruit stems to eliminate overwintering sites for fungi.
Bitter rot

(Bill Cline, NCSU)
Diagnostic features: Black acervuli covering berries
Pathogen: Melaconium fuligineum
Comments: Remove fallen debris and mummified fruit; during the winter, remove all old fruit stems to eliminate overwintering sites for fungi.
Ripe rot

(Bill Cline, NCSU)
Diagnostic features: Dark brown rot with pink masses of spores covering part or all of fruit
Pathogen: Glomerella cingulata - see Apple bitter rot
Comments: Remove fallen debris and mummified fruit. During the winter, remove all old fruit stems to eliminate overwintering sites for fungi.
Macrophoma rot

(Bill Cline, NCSU)
Diagnostic features: Small, sunken, black fruit spots — round with distinct edges
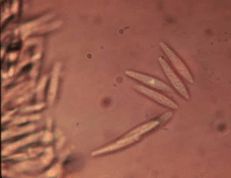
Conida
Pathogen: Botryosphaeria dothidea
Comments: Remove fallen debris and mummified fruit. During the winter, remove all old fruit stems to eliminate overwintering sites for fungi.
Angular leaf spot

(Bill Cline, NCSU)
Diagnostic features: Light yellow spots; irregular brown flecks develop in the center
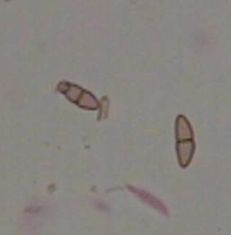
Ascospores
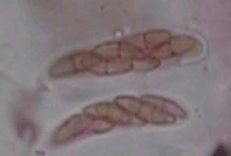
Asci
Pathogen: Mycosphaerella angulata
Comments: This pathogen can cause leaf spotting that can lead to rapid defoliation. Remove fallen debris and mummified fruit; during the winter, remove all old fruit stems to eliminate overwintering sites for fungi. Pruning is equally important.
Powdery mildew

(Bill Cline, NCSU)
Diagnostic features: Surface russeting on fruit
Pathogen: Uncinula necator - see Grape powdery mildew
Comments: Attacks young berries — causes a russetted look. Berry drop and reduced size result from infections. Improve air circulation and use proper sanitation practices
Peaches, Nectarines, and Plums
Brown rot

Diagnostic features: Masses of conidia covering light brown fruit rot

Apothecia (sexual fruiting structure) on a peach mummy
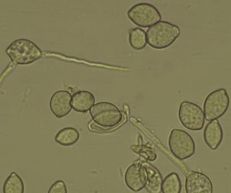
Conidia
Pathogen: Monilinia fructicola
Comments: Major disease of these fruits in Georgia. Disease infects blooms, stems and fruit. Sanitation is the key! Remove and/or prune infected tissues and areas on trees. Remove and discard mummies. During wet summers, green fruit injured by insects and/or environment will develop brown rot. Remove any fruit that has fungal growth.
Peach scab
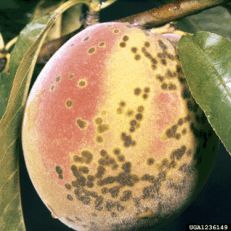

Diagnostic features: Raised dark brown lesions on twigs; greenish brown-black lesions covering fruit, sometimes surrounded by yellow halo
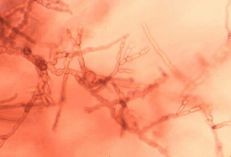
Conidiophores and conidia
Pathogen: Cladosporium carpophilum
Comments: Disease infects both fruit and twigs of current years' growth. Small, oval to round, gray to black spots on fruit. Fruit may crack because of coalescing of scabs. Pruning trees to promote penetration of sunlight and air circulation is necessary.
Gummosis

Diagnostic features: Gum/jelly produced on trunk
Conidia
Pathogen: Botryosphaeria dothidea
Comments: Disease causes sunken lesions with oozing amber-colored resin or gum on trunks, limbs, and twigs. Small twigs may be killed as disease progresses. Prune out and remove dead wood. Irrigation during periods of dry weather helps to reduce plant stress and may minimize disease.
Peach leaf curl

Diagnostic features: Deformed leaves (wrinkled, puckered, and/or curled)
Pathogen: Taphrina deformans
Comments: Disease occurs in cooler areas of the state — primarily the upper Piedmont and mountains. Apply this to nectarines and peaches only. Sanitize. For successful control, the fungicides must be applied before bud swell.
Strawberries
Leaf spots

(Courtesy Jeff Cook)

Diagnostic features: Leaf spot (gray/white center with purple border) – Mycosphaerella sp.; Angular Leaf Spot (restricted by veins) – Xanthomonas sp.

Angular Leaf spot — Bacterial Streaming (@ 40X)
Pathogen: Mycosphaerella fragariae; Xanthomonas sp.
Comments: Leaf spot — upper leaf surface first as tiny, round, purple spot 1/8" in diameter; spot becomes gray with purple border. Loss of foliage is common.
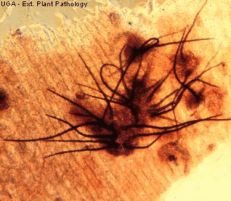
Anthracnose

(Courtesy Tom Jennings)
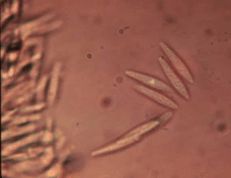
Diagnostic features: Light to dark brown sunken lesions on fruit
Long, black setae
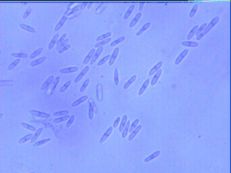
Conidia
Pathogen: Colletotrichum sp.
Comments: Anthracnose is a major disease of strawberries when conditions are wet. Anthracnose infects stolons, petioles, crowns, fruit and leaves. Small dark lesions form on stolons and petioles in summer. Crowns can be infected, resulting in plant death. Remove infected plants or fruit and destroy or bury. Always purchase disease-free plants!
Botrytis blight

Diagnostic features: Botrytis blight on strawberry fruit


Conidiophores and conidia
Pathogen: Botrytis cinerea
Comments: Botrytis is often present in strawberry leaves, etc., even if symptoms are not present. These quiescent infections give rise to production of spores under wet conditions during bloom. Blossoms need to be protected season-long to reduce fruit infection and to prevent epidemic development.
Rhizoctonia root and crown rot

Robust, septate, pigmented, branching mycelia
Pathogen: Rhizoctonia spp.
Comments: Root rot is favored by cool weather, but crown rot is worse in hot weather. Plants start collapsing as fruiting starts. The bottom of the leaves are purple and leaves curl up as the original crown is killed. Buy diseasefree plants.
Phomopsis leaf blight

Diagnostic features: V-shaped leaf lesions progressing from leaf margin to leaf interior

Conidia (2 types)
Pathogen: Phomopsis obscurans
Comments: Disease starts in the fall or spring after planting. It spreads rapidly and can destroy most of the foliage. Remains active as long as there is green foliage. Symptoms occur as circular red to purple spots on leaflets; the spots enlarge and develop gray centers, making large V-shaped lesions. Fruit and calyx infection also occurs. Remove infected foliage. Fruit infection is prevented by controlling foliar infection.
References
Literature:
Brannen, P. M. (2022). Orange felt (Orange cane blotch) of blackberry (Publication No. C 892). UGA Cooperative Extension. https://extension.uga.edu/publications/detail.html?number=C892
Brannen, P. M., & Krewer, G. (2023). Cane blight of blackberry (Publication No. C 894). UGA Cooperative Extension. https://extension.uga.edu/publications/detail.html?number=C894
Eaker, T. H. (2002). Sanitation measures for limiting diseases in the home orchard.
Maas, J. L. (1998). Compendium of strawberry diseases (2nd ed.). APS Press. https://apsjournals.apsnet.org/doi/book/10.1094/9780890546178
Sial, A., Taylor, M. D., & Cabrera, E. (Eds.). (2022). Georgia pest management handbook — Home and garden edition (Publication No. SB 48). University of Georgia Cooperative Extension. https://extension.uga.edu/publications/detail.html?number=SB48
Southern Region Small Fruit Consortium. (n.d.). IPM/production guides. https://smallfruits.org/ipm-production-guides/
Images:
UGA1236183 — Clemson University - USDA Cooperative Extension Slide Series.
UGA1436071 — Clemson University - USDA Cooperative Extension Slide Series.
UGA1234176 — Clemson University - USDA Cooperative Extension Slide Series.
UGA5026050 — Jody Fetzer, New York Botanical Garden.
UGA4213005b — Minnesota Department of Natural Resources Archives, Minnesota Department of Natural Resources.
UGA1234145 — Clemson University - USDA Cooperative Extension Slide Series.
UGA4823038 — Edward L. Barnard, Florida Department of Agriculture and Consumer Services.
UGA1496476 — UGA Plant Pathology Archives.
UGA1436096 — Clemson University - USDA Cooperative Extension Slide Series.
UGA1436097 — Clemson University - USDA Cooperative Extension Slide Series.
UGA1436095 — Clemson University - USDA Cooperative Extension Slide Series.
UGA1495075 — UGA Plant Pathology Archives.
UGA0176033 — M. Clerjeau, INRA, Centre de Recherches de Bordeaux.
UGA1436098 — Clemson University - USDA Cooperative Extension Slide Series.
UGA0162045 — Jack Clark, University of California-Davis.
UGA1495106 — UGA Plant Pathology Archives.
UGA1495099 — UGA Plant Pathology Archives.
UGA5077075 — David Langston, UGA.
UGA0176035 — M. Clerjeau, INRA, Centre de Recherches de Bordeaux.
UGA1436094 — Clemson University - USDA Cooperative Extension Slide Series.
UGA1436082 — Clemson University - USDA Cooperative Extension Slide Series.
UGA1236149 — Clemson University - USDA Cooperative Extension Slide Series.
UGA1492030 — UGA Plant Pathology Archives.
UGA1436090 — Clemson University - USDA Cooperative Extension Slide Series.
UGA1496549 — UGA Plant Pathology Archives.
Images listed above are available at www.forestryimages.org. Unlabeled images are from the Extension Plant Pathology Archives, Phil Brannen, or Holly Thornton.
Status and Revision History
Published on Feb 14, 2008
Published on Oct 09, 2009
Published on Feb 18, 2011
Published with Full Review on May 29, 2015
Published with Full Review on May 10, 2023
