A critical step in the National Mastitis Council’s recommended milking routine (Figure 1) is checking foremilk—to look for signs of mastitis and to maintain milk quality—and stimulating the teats in preparation for milk collection.

Figure 1. National Mastitis Council’s Best Milking Practices.
Adapted from “Recommended milking procedures,” by the National Mastitis Council, 2013, http://www.nmconline.org/wp-content/uploads/2016/09/Recommended-Milking-Procedures.pdf
Forestripping is removing the foremilk, which is the first three to five streams of milk. During the premilking routine it serves three important purposes:
- teat stimulation, which results in optimal milk release;
- enhanced milk quality by removing milk with the highest bacterial and somatic cell count (SCC);
- identification of mastitis, to allow rapid, well-informed decision-making regarding further evaluation, milk culturing, and/or antibiotic therapy.
This publication will discuss the purposes of forestripping and serve as a resource for producers when creating and evaluating best milking practices.
Teat Stimulation
Stimulation of the teats for 10–15 s results in optimal milk released from the alveoli, the milk-producing units of the mammary gland. The events leading up to and including milk release are part of a process called milk letdown. A basic understanding of milk letdown prompted by teat stimulation is critical to appreciate its importance for milk quality. The steps of milk letdown induced by teat stimulation are numbered in Figure 2 and include:
- The teat is physically stimulated (e.g., by suckling or touch).
- Messages travel to the brain through nerves.
- The hormone oxytocin is released from the posterior pituitary gland.
- Oxytocin travels to the mammary gland in the blood.
- Oxytocin binds to smooth muscle cells, known as myoepithelial cells, surrounding the alveoli to cause squeezing, or letdown, of milk for collection.
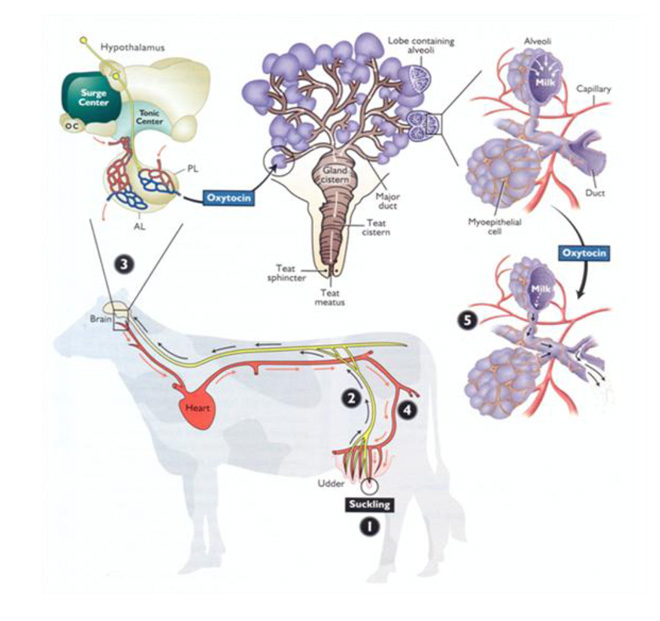
Figure 2. An illustration of the milk letdown pathway.
From “Pathways to pregnancy and parturition” (2nd ed.), by P. L. Senger, 2003. Copyright 2003 by Current Conceptions, Inc.
There is an approximately 1- to 2-min lag between teat stimulation and milk letdown. The effect of oxytocin on myoepithelial cells of the alveoli lasts 5 to 7 min on average but is strongest in the first 3 min. For these reasons and to maximize the benefits of optimal teat stimulation, the milking unit should be attached within 2 min of the initial touch.
Though poor teat stimulation does not consistently decrease the total milk yield possible in a single milking event, milk flow will be interrupted—contributing to overmilking and damage to the teat ends and internal mammary tissues. Interrupted milk flow happens after the residual cistern milk (teat and gland, Figure 3) has been removed before milk letdown is activated from the deeper portions of the mammary tissue. The teat and gland cisterns of the four separate quarters hold about 20% of the milk collected during a milking event. Interrupted milk flow can be observed visually by monitoring the outflow of milk or with inline milk sensors that provide milk flow rates. Bimodal milk flow (Figure 4), where there are two distinct peaks in milk flow, indicates interrupted milk flow. The first peak in a bimodal curve represents milk present in the teat and gland cisterns and the second peak indicates when full milk letdown begins. Bimodal milk flow results in overmilking, which contributes to teat end and mammary tissue damage.
Teat-end damage as a result of overmilking is visible as hyperkeratosis, the thickening of the outermost teat skin layer. This thickening is a result of increased keratin, visualized in Figure 5 as the ragged edges of the teat end (darker pink), where normally this area should be smooth. Figure 6 is an image of a cow’s teat suffering from hyperkeratosis, indicated by the raised ridges on the ring of the teat end. Hyperkeratosis can negatively impact milk letdown because sensitive nerves necessary for proper teat stimulation are located at the teat end. Hyperkeratosis could result in improper closure of the teat sphincter muscle, one of the most important defense mechanisms against bacterial entry into the mammary gland (see Figure 3). If you suspect hyperkeratosis is a problem, a scorecard (Figure 7) can be used in teat-end assessments. A score of 1 indicates a healthy teat with no ring and no raised edges, while a score of 4 indicates a raised ring and very rough edges, or 5 indicates open lesions on the teat. The cracks and cervices created by hyperkeratosis provide an area for bacteria to colonize and make cleaning and disinfection difficult, which increases the risks of mastitis and high SCC.
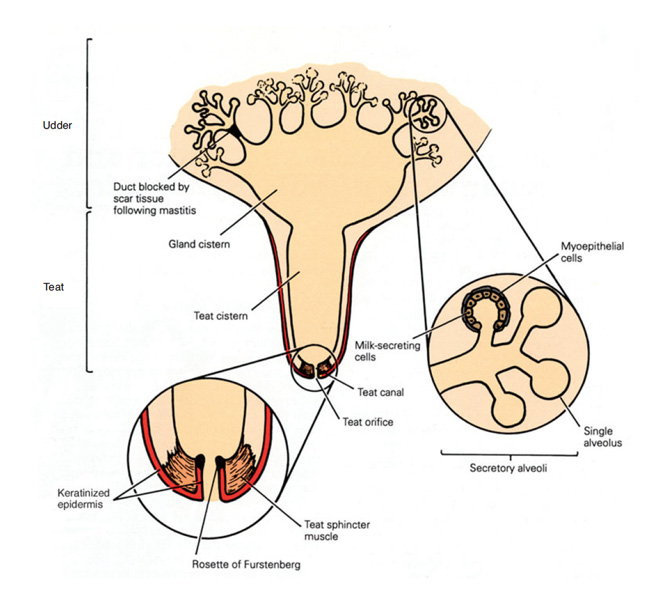
Figure 3. Anatomy of the mammary gland.
From “Mastitis control in dairy herds” (2nd ed.), by R. Blowey, and P. Edmondson, 2010. Copyright 2010 by CABI.
Even with proper teat stimulation for 10–15 s, environmental factors can negatively affect milk letdown. The flight-or-fight response is the primary event that can negatively impact milk letdown. Fear, pain, or stress may activate the fight-or-flight response, which results in blood being sent to the limbs rather than to the mammary gland; this effect can last for up to 30 min. Remember that the hormone responsible for milk letdown, oxytocin, must travel in the blood to get to the mammary gland. Ensure a calm, stress-free trip to the parlor by avoiding loud noises or shouting, frequent changes in routine, prods or sticks, and rough handling.
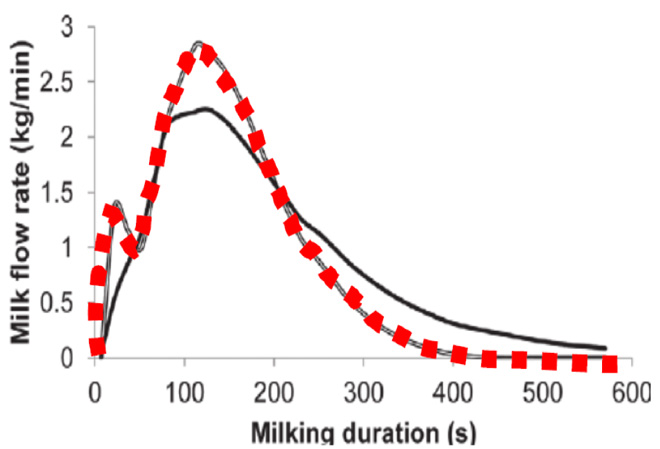
Figure 4. Milk flow rates (kg/min) during proper milk flow (black solid line) compared to bimodal flow (red dashed line).
From “Analysis of milking characteristics in New Zealand dairy cows,” by P. Edwards, J. G. Jago, and N. Lopez-Villalobos, 2013, Journal of Dairy Science, 97(1), p. 266 (https://doi.org/10.3168/jds.2013-7051). Copyright 2014 by the American Dairy Science Association.
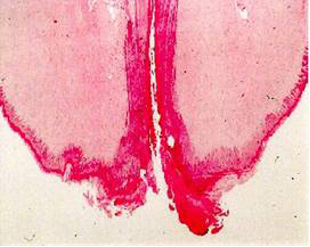
Figure 5: Hyperkeratosis at the teat end.
Photo: A. de Man, Y. H. Schukken, and J. P. Koeman, Utrecht University.
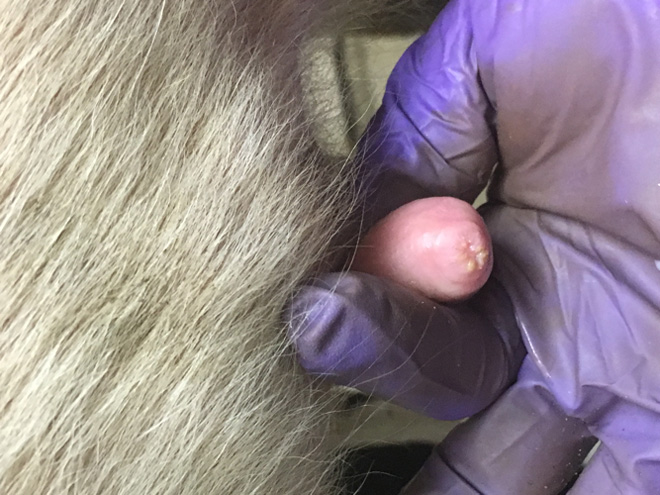
Figure 6: Hyperkeratosis at the teat end. Notice the raised edges at the teat end.
| Score | Description | Illustration |
|---|---|---|
| 1 |
No ring The teat-end is smooth with a small, even orifice. This is a typical status for many teats soon after the start of lactation. |
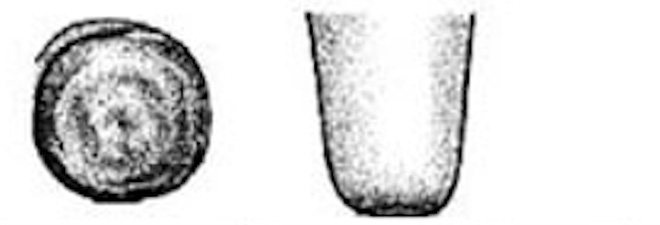 |
| 2 |
Smooth or Slightly rough ring A raised ring encircles the orifice. The surface of the ring is smooth or it may feel slightly rough but no fronds of old keratin are evident. |
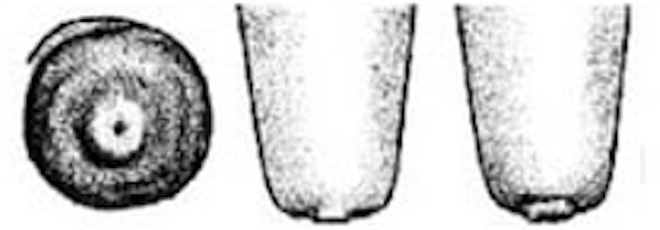 |
| 3 |
Rough ring A raised, roughened ring with isolated fronds or mounds of old keratin extending 1 - 3 mm from the orifice. |
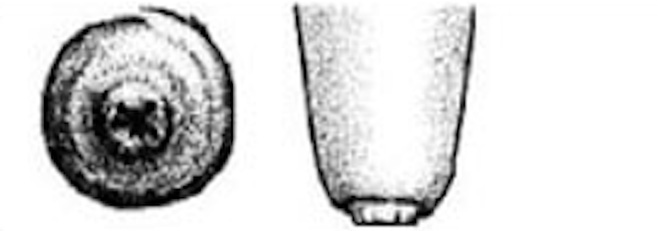 |
| 4 |
Very Rough ring A raised ring with rough fronds or mounds of old keratin extending 4 mm or more from the orifice. The rim of the ring is rough and cracked, often giving the teat-end a “flowered” appearance. |
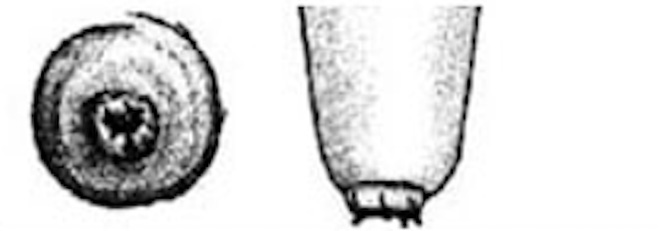 |
| 5 |
Open Lesions or Scabs Teat end is severely damaged and ulcerative with scabs or open lesions. |
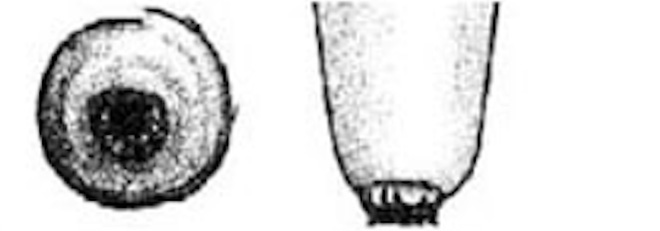 |
Figure 7: Teat-end condition scoring chart.
Adapted from “Evaluation of bovine teat condition in commercial dairy herds: 1. Non-infectious factors,” by G. A. Mein, F. Neijenhuis, W. F. Morgan, D. J. Reinemann, E. Hillerton, J. Baines, I. Ohnstad, M. D. Rasmussen, L. L. Timms, J. S. Britt, R. Farnsworth, and N. Cook, 2001, Proceedings of the AABPNMC International Symposium on Mastitis and Milk Quality, p. 10 (https://www.researchgate.net/publication/237767923_Evaluation_of_Bovine_Teat_Condition_
in_Commercial_Dairy_Herds_1_Non-Infectious_Factors). Copyright 2001 Mein et al.
Enhance Milk Quality
Stripping foremilk removes the milk that is highest in bacterial and somatic cell counts in the mammary gland. Increased bacteria and SCC in milk negatively affect milk quality and ultimately the economic success of an operation. Elevated levels of bacteria and somatic cells are detrimental not only in mastitic cows but also can create issues in healthy cows. Understanding the causes of and ways to control both SCC and bacterial counts will help producers improve udder health.
Bacteria in Milk
Some of the first published reports of greater bacterial numbers in the foremilk date back to the late 1800s and into the early 1900s. Some of the additional findings include: (a) increased bacteria in foremilk was unrelated to whether a mammary infection existed, (b) as cows aged the number of bacteria in foremilk increased compared to younger animals, and (c) discarding the first five streams of milk was sufficient to reduce the bacterial number per milliliter of milk to a point where it was similar to bacterial numbers in midflow milk. Other studies also found the highest amounts of bacteria in the first three to five streams of milk.
Bacteria in milk is assessed by milk cooperatives and processors to determine whether milk is acceptable for processing. The three measures of bacterial content in milk include:
- Standard plate count (SPC): The raw bacterial count, or SPC, reflects the total bacteria in milk and, according to the Pasteurized Milk Ordinance, must be below 100,000 colony forming units (cfu) per milliliter. However, cooperatives and processors typically have a goal of < 10,000 cfu/ml, as bacteria affect manufacturing and shelf life of dairy products.
- Preliminary incubation count (PIC): Most bacteria, especially most of those that cause mastitis, grow near or at body temperature (98.6 °F). To determine PIC, the milk is incubated at 55 °F for 18 hr prior to counting bacteria. Thus, the PIC indicates the number of bacteria that are able to grow at cooler temperatures. The concern is that, even with the milk being cooled, these bacteria can grow in the bulk tank and reduce shelf life.
- Laboratory pasteurization count (LPC): The LPC is determined by performing a standard pasteurization procedure to kill most bacteria, especially those that cause mastitis. Bacteria that survive this process can end up in dairy products and negatively affect the product.
While there are no legal standards for PIC and LPC, there are general benchmarks to use to assess mammary health, milking practices, and milk parlor cleaning procedures. The PIC should be below 50,000/ml and the LPC should be below 100–200/ml. Additionally, the SPC, PIC, and LPC can be used to identify areas of possible bacterial contamination (Table 1). More information can be found at www.nmconline.org.
Table 1. Possible sources of milk bacterial contamination in dairy operations.
| Test result | Natural flora of teat skin | Mastitis | Dirty cows | Dirty equipment | Poor cooling |
|---|---|---|---|---|---|
| SPC > 10,000 | Unlikely | Possible | Possible | Possible | Possible |
| SPC > 10,000 | Unlikely | Possible (rare) | Unlikely | Very likely | Very likely |
| LPC > 200–300 | Unlikely | Unlikely | Possible | Very likely | Unlikely |
| Higher PIC than SPC | Unlikely | Unlikely | Possible | Very likely | Very likely |
| Higher SPC than PIC | Unlikely | Possible | Possible (not likely) | Possible (not likely) | Possible (not likely) |
| Coliform | Unlikely | Possible (rare) | Possible | Possible | Possible (not likely) |
Note. Table is compiled from information in Murphy & Boor (2019). SPC = standard plate count; LPC = laboratory pasteurization count; PIC = preliminary incubation count.
Somatic Cell Count in Milk
Somatic cells are present in the foremilk of both healthy and mastitic cows. In healthy cows without mastitis, hundreds to thousands of somatic cells can be concentrated in the foremilk. One study reported that the foremilk represented 20% of the total SCC of milk. If your cell count is 250,000 cells/ml, removing the foremilk could reduce your overall SCC for that animal to 200,000 cells/ml or below. Increased foremilk SCC was especially observed in cows with elevated SCC. In cows that had a total SCC of over 500,000/ml, the foremilk fraction had a SCC ranging from 1,500,000 to 2,500,000/ml.
In another study, Wellnitz et al. (2009) were unable to use foremilk SCC for estimating total quarter SCC because foremilk SCC was so high. These researchers noted that not only is removing this portion of milk important for reducing overall SCC as it enters the bulk tank, but also removing foremilk for evaluation of subclinical mastitis may be even more important to operations. An accurate SCC will allow producers to fully evaluate the milk loss attributed to high SCC and determine if they have subclinical infections that should be further investigated (Table 2).
Table 2. Estimating production lost and percent of infected quarters in a herd by bulk milk somatic cell counts.
| Bulk tank SCC | Percent infected quarters in herd |
Percent production loss |
|---|---|---|
| 200,000 | 6 | 0 |
| 500,000 | 16 | 6 |
| 750,000 | 25 | 12 |
| 1,000,000 | 32 | 18 |
| 1,5000,000 | 48 | 29 |
Note. Table is compiled from information in Murphy & Boor (2019). SPC = standard plate count; LPC = laboratory pasteurization count; PIC = preliminary incubation count.
Identify Clinical Mastitis
The third primary reason to include forestripping in your milking routine is to identify clinical mastitis and other abnormalities in the teat and udder. While the udder may become red and swollen during some cases of mastitis, the most common physical change is milk’s appearance. Mastitic milk may be off-color, bloody or include blood clots, have a watery consistency, or exhibit flakes, clumps, and stringiness. Assessing foremilk for signs of mastitis will prevent delayed antibiotic treatment, allow for quicker culture of mastitis-causing pathogens, and/or overall enable more rapid management decisions. A delay in identifying mastitis results in increased losses in milk production from the infection. Forestripping is a more effective method for quickly identifying clinical mastitis than observation of the udder for abnormalities.
Newer technologies that allow for in-line assessment of milk can be useful in conjunction with visual assessment. As a result of the breakdown in the blood-milk barrier during mastitis, ions (e.g., sodium) increase in milk, which changes its electrical conductivity (EC). The change in EC occurs in the very early stages of mastitis, even before increased SCC or changes in milk appearance in many cases. If EC data is monitored and managed well, EC can be a valuable tool in early detection of mastitis. Caution must be taken when evaluating EC, however, because different pathogens result in varying changes in EC, cows and herds have different baselines and threshold changes, and tremendous overall variation may make decision-making challenging. There are handheld cow-side EC devices that also can be used, but the same cautions would apply. Unfortunately, without additional diagnostics (SCC, milk culture, etc.), a high number of false positives typically are detected. Current recommendations suggest that changes in milk EC can serve as a screening tool to determine which animals need further evaluation, such as stripping to observe appearance, assessment of SCC, or milk culturing. Other methods of assessment that do not require visual assessment of milk include changes in milk pH and changes in milk enzymes, but these assessments require the same cautions as those mentioned for EC.
Summary
Forestripping the first three to five streams of milk as part of the premilking routine serves three important purposes: (a) teat stimulation to maximize milk release, (b) enhancement of milk quality by removing the milk with the highest bacterial and somatic cell counts, and (c) identification of mastitis to promote rapid, well-informed decisions (further evaluation, milk culturing, and/or antibiotic therapy).
Incorporating forestripping into an effective premilking procedure can improve udder health, milk production, and ultimately improve the profitability of producers. For more information, contact your local Extension Office: visit extension.uga.edu or call 1-800-ASK-UGA-1.
References
Blowey, R., & Edmondson, P. (2010). Mastitis control in dairy herds (2nd ed.). CABI. https://www.lactoscan.com/editor/ufo/manuals/ SCC/Mastitis_Control_in_Dairy_Herds_2nd(veterinary-student.blogfa.com).pdf
Bruckmaier, R. M., Schams, D., & Blum, J. W. (1994). Continuously elevated concentrations of oxytocin during milking are necessary for complete milk removal in dairy cows. Journal of Dairy Research, 61(3), 323–334. https://doi.org/10.1017/S0022029900030740
Bruckmaier, R. M., & Blum, J. W. (1998). Oxytocin release and milk removal in ruminants. Journal of Dairy Science, 81(4), 939–949. https://doi.org/10.3168/jds.S0022-0302(98)75654-1
Edwards, P., Jago, J. G., & Lopez-Villalobos, N. (2013). Analysis of milking characteristics in New Zealand dairy cows. Journal of Dairy Science, 97(1), 259–269. https://doi.org/10.3168/jds.2013-7051
Faber, J. (1930). A study of the bacterial content of the foremilk of cows. Journal of Dairy Science, 13(6), 449–452. https://doi.org/10.3168/jds.S0022-0302(30)93544-8
Findlay, A. L. R. (1966). Sensory discharges in lactating mammary glands. Nature, 211, 1183–1184. https://doi.org/10.1038/2111183a0
Guarin, J. F., Paixao, M. G., & Ruegg, P. L. (2017). Association of anatomical characteristics of teats with quarter-level somatic cell count. Journal of Dairy Science, 100(1), 643–652. https://doi.org/10.3168/jds.2016-11459
Sarikaya, H., & Bruckmaier, R. M. (2006). Importance of the sampled milk fraction for the prediction of total quarter somatic cell count. Journal of Dairy Science, 89(11), 4246–4250. https://doi.org/10.3168/jds.S0022-0302(06)72470-5
Martins, S. A. M., Martins, V. C., Cardoso, F. A., Germano, J., Rodrigues, M., Duarte, C., Bexiga, R., Cardoso, S., & Freitas, P. P. (2019). Biosensors for on-farm diagnosis of mastitis. Frontiers in Bioengineering and Biotechnology, 7, 186. https://doi.org/10.3389/fbioe.2019.00186
Mein, G. A., Neijenhuis, F., Morgan, W. F., Reinemann, D. J., Hillerton, J. E., Baines, J. R., Ohnstad, I., Rasmussen, M. D., Timms, L., Britt, J. S., Farnsworth, R., & Cook, N. B. (2001, September 13–15). Evaluation of bovine teat condition in commercial dairy herds: 1. Non-infectious factors [Paper presentation]. AABP-NMC International Symposium on Mastitis and Milk Quality, Vancouver, Canada. https://www.researchgate.net/publication/237767923_Evaluation_of_Bovine_Teat_Condition_in_Commercial_Dairy_ Herds_1_Non-Infectious_Factors
Murphy, S.C., & Boor, K. J. (2019). Sources and causes of high bacteria counts in raw milk: An abbreviated review. Retrieved February 12, 2020, from https://dairy-cattle.extension.org/sources-and-causes-of-high-bacteria-counts-in-raw-milk-an-abbreviated-review/
National Mastitis Council. (2013). Recommended milking procedures. Retrieved March 26, 2020, from http://www.nmconline.org/wpcontent/uploads/2016/09/Recommended-Milking-Procedures.pdf
Norberd, E. (2005). Electrical conductivity of milk as phenotypic and genetic indicator of bovine mastitis: A review. Livestock Production Science, 96(2–3), 129–139. https://doi.org/10.1016/j.livprodsci.2004.12.014
Paduch, J. H., Mohr, E., & Kromker, V. (2012). The association between teat end hyperkeratosis and teat canal microbial load in lactating dairy cattle. Veterinary Microbiology, 158(3–4), 353–359. http://doi.org/10.1016/j.vetmic.2012.02.032
Senger, P. L. (2003). Pathways to pregnancy and parturition (2nd ed.). Current Conceptions, Inc.
Tikofsky, L. (2011). Subclinical mastitis — What you can’t see CAN hurt you. NODPA. Retrieved May 5, 2020, from https://nodpa.com/n/463/Subclinical-Mastitis--What-You-Cant-See-CAN-Hurt-You
Wellnitz, O., Doherr, M. G., Woloszyn, M., & Bruckmaier, R. M. (2009). Prediction of total quarter milk somatic cell counts based on foremilk sampling. Journal of Dairy Research, 76(3), 326–330. https://doi.org/10.1017/S0022029909004166
Status and Revision History
Published on May 22, 2023
