- Forage Quality Has Value
- The Need for Forage Testing
- Obtaining a Representative Forage Sample
- Measures of Forage Quality
- Near Infrared Spectroscopy
- Simplifying Forage Quality Assessments
- Reading a Forage Quality Analysis
- Management Factors Affect Forage Quality
- Highlights of a Forage Quality Analysis Report
- Forage Quality of Major Southern Forages: Summary Statistics
- Digestibility Sometimes Differs Between Varieties
- Summary
- Related Publications and Resources
The nutritive value of a specific lot of forage is defined by the amount of nutrition that can be derived from it and the presence/concentration of any toxic compounds that could reduce animal performance or threaten animal health. In combining the nutritive value of the forage with assumptions/predictions of how much of the forage an animal could eat, one can determine if the forage's quality is sufficient. It is important to understand the quality of the forage being used so as to develop a least-cost ration for the animals being fed. Estimates of protein, mineral, and vitamin content can be made relatively easily. However, the majority of the available energy in a forage crop is in a fibrous form. Several analytical procedures have been developed to provide estimates of fiber content and digestibility. These estimates have also been calibrated to predict animal nutrition and performance.
The goal of this publication is to guide the user to a better understanding of basic forage quality terms and to recommend management changes that will improve forage quality. To that end, our objectives are to explain how forage quality is measured, describe how to interpret a forage analysis, present the effects of management on forage quality, and list the key management strategies that can increase the nutritive value of forage crops. This publication is written with the understanding that the reader either knows or can quickly find the definition of key forage quality terms. The reader is encouraged to refer to the glossary of UGA Extension Bulletin 1367, “Common Terms Used in Animal Feeding and Nutrition,” for unfamiliar terms used in this publication.
Forage Quality Has Value
Commodity and by-product feeds are relatively expensive. Certainly, providing high-quality forage (either as pasture, hay, baleage/haylage, or silage) is not inexpensive, either. With recent feed prices, however, high-quality forage is cheaper than most supplements that are typically fed. Forage that is lower in digestibility will not meet the nutrient requirements of the animal requiring supplementation, which increases the cost of production. As a result, more and more supplement is needed to meet the requirements of the animal (Table 1).
Table 1. The effect of bermudagrass and tall fescue maturity on hay quality, supplementation rate, and cost of supplementing a lactating beef cow.1
| Crop | Maturity | Crude Protein (CP) |
Total Digestible Nutrients (TDN) |
Supplement Req. for a Lact. Beef Cow |
Cost to Supplement |
| ---- % ---- | ---- % ---- | lbs/hd/day | $/hd/day | ||
| Bermudagrass | 4 weeks | 10-12 | 58-62 | 0 | $0 |
| 6 weeks | 8-10 | 51-55 | 2.3 – 4.8 | $0.23 – 0.48 | |
| 8 weeks | 6-8 | 45-50 | 5.3 – 7.5 | $0.53 – 0.75 | |
| Tall Fescue | Late boot | 14-16 | 66-70 | 0 | $0 |
| Early head | 11-13 | 60-63 | 0 | $0 | |
| Dough (seed) | 8-10 | 50-54 | 3.0 - 5.3 | $0.30 – 0.53 | |
| 1 Assumptions: 1,200 lb beef cow, average to above-average milking ability, first three months postpartum, 6.0 lbs of TDN required daily, and supplement that provides 85% TDN and costs $200/ton ($0.10/lb). | |||||
The Need for Forage Testing
There is no technique for assessing the nutritional value of the forage in a pasture or lot of hay or silage strictly on the basis of feel, texture, smell, or appearance. In fact, attempting to do so has frequently caused producers to buy or use forage that has lower nutritional value and is often uneconomical or counterproductive (Figure 1). The nutritional value of the forage can only be evaluated by obtaining a representative sample of the forage and subjecting that sample to analysis in a qualified laboratory.
 Figure 1. Though different in appearance, lots 1 and 2 are essentially the same quality.
Figure 1. Though different in appearance, lots 1 and 2 are essentially the same quality. Figure 2. Sampling for forage quality can aid decisions, such as storage priority.
Figure 2. Sampling for forage quality can aid decisions, such as storage priority.
The results of a forage test can be used for a number of purposes. One of the most important uses is to formulate cost-effective rations. Forage testing is also used to establish the nutritive and, therefore, market value of the lot. Though hay, silage, and other conserved forages have not typically been marketed in the Southeast on the basis of nutritive quality, savvy producers are increasingly insisting upon having a forage quality analysis for any lots that they may be purchasing. Another purpose for sampling forage is to prioritize the lots that one may have in inventory for timely use (Figure 2). For example, a beef cow-calf producer with limited hay storage space may want to store their low-quality forage outside while storing the higher quality lots under cover so as to better protect the more valuable forage.
Obtaining a Representative Forage Sample
 Figure 3. Sampling a lot of hay bales using a Colorado hay probe.
Figure 3. Sampling a lot of hay bales using a Colorado hay probe.Obtaining a representative forage sample is critical. The first step is to identify a single lot (forage taken from the same farm, field, and cut under uniform conditions within a 48-hour time period). Once a lot is defined, sub-samples should be obtained from at least 20 different bales (hay, baleage) or areas (silage) that are selected at random. Detailed procedures for sampling forage are provided by the National Forage Testing Association (http://www.foragetesting. org/). Avoid taking grab samples from the bale or stack, as this may cause leaf loss and result in a sample that is not a fair representation of the lot. It is best to use a clean, sharp, forage probe (Figure 3). For information on selecting and purchasing forage probes, see the frequently asked question page titled “What hay probe do you recommend and where can I get one?” (http://www.caes.uga. edu/commodities/fieldcrops/forages/questions/hayprobes.html) on the University of Georgia's Forage Extension website (www.georgiaforages.com).
Measures of Forage Quality
Certainly, the ultimate evaluation of forage quality is animal performance. But, it is obviously not practical to do feeding trials to estimate the quality of each lot of forage crop. Thus, predictions of forage digestibility and rate of intake have been developed that allow one to better estimate the forage crop's nutritive value.
Predictions of forage digestibility and intake rate are based on the fibrous part of the forage. Since a forage crop is essentially a collection of many plant cells, a simplified example of a single plant cell can help to illustrate the various fractions and components (Figure 4).
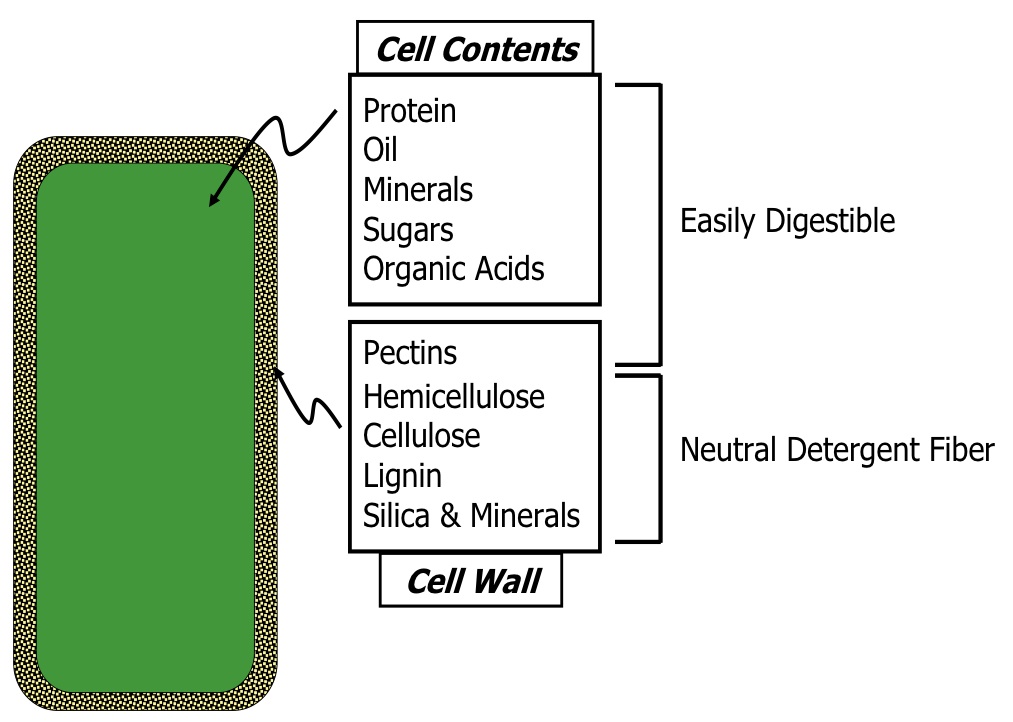 Figure 4. The easily digestible components of a cell and the fibrous components (NDF) of the cell wall.
Figure 4. The easily digestible components of a cell and the fibrous components (NDF) of the cell wall.A plant cell is made up of a cell wall and cell contents (i.e., material inside the cell). Essentially, all of the cell contents are easily digestible. However, the cell wall is fibrous and less digestible. This fibrous fraction can be measured and divided into components using a stepwise laboratory procedure. This procedure involves the extraction of the cell components in a progressive manner, starting with the most easily removed components (cell contents and pectins). The soluble cell contents and the pectin within the cell wall are removed by boiling the sample in a neutral detergent. The whole fibrous fraction that remains is known by the first step in the chemical analysis, neutral detergent fiber (NDF) analysis. This NDF fraction consists of hemicellulose, cellulose, lignin, and silica/minerals. The next step involves boiling the NDF fraction in an acid detergent, which dissolves and washes away the hemicellulose component. This leaves the acid detergent fiber (ADF) fraction, which consists of cellulose, lignin, and silica/minerals. Next, the ADF fraction is further treated with a stronger acid to dissolve the cellulose to leave just the lignin and silica/mineral components. Finally, the remaining fraction is burned in a 500° C furnace, leaving just the silica/mineral components in the ash.
The concentration of NDF and the ratios of the subcomponents of NDF in relation to one another have direct effects on the digestibility of the forage. Cellulose (a long chain of glucose molecules linked end to end) and hemicellulose (a branched polymer of glucose, xylose, galactose, and other carbohydrates) can be broken down by enzymatic action of bacteria and other microbes in the animal's digestive tract, though their digestion is markedly slower than the digestion of sugars, starches, and other freely available non-structural carbohydrates. In contrast, lignin is not carbohydrate-based but is a phenolic compound. As such, lignin is not digestible. Moreover, the very presence of lignin acts as a physical barrier to the microbial enzymes that break down cellulose and hemicellulose. As a result, the amount of NDF and proportion of the NDF that is hemicellulose, cellulose, lignin, and silica/mineral are known to influence and can be used to estimate other aspects of forage quality. It is those components that are used to calculate metrics like total digestible nutrients (TDN), metabolizable energy (ME), and net energy for maintenance (NEm), gain (NEg), and lactation (NEl)1. These variables, along with a measure of crude protein (CP) and mineral content, can then be used to develop a balanced ration that meets the nutritional needs of the animal type/class.
1 It is important to note that the estimates of TDN, ME, NEm, NEg, and NEl are made using predictions specific to the animal species and class that is being fed.
In addition to ration balancing, the results of a forage analysis can be helpful to identify nutritional problems or toxin-related disorders. Frequently, a ration may be balanced for CP but not supply enough energy or mineral content. Furthermore, some measures of forage quality can be used to estimate dry matter (DM) intake by the livestock class being fed. An overview of the important uses of the forage quality metrics specified on reports from the University of Georgia's Feed and Environmental Water Laboratory are presented in Table 2.
Table 2. Summary of the primary uses of the forage quality metrics specified on reports from the University of Georgia's Feed and Environmental Water Laboratory.
| Important Uses | |||||||
| Metric | Abbrev. | Units | Analytical Method |
Ration Balancing |
Nutritional Diagnostics |
Energy Estimates |
Involved in Estimating DM Intake |
| Standard Procedures | |||||||
| Relative Forage Quality2 | RFQ | -- | NIR | ||||
| Crude Protein | CP | % | NIR, WC | x | x | x | |
| Crude Fiber3 | CF | % | NIR | ||||
| Neutral Detergent Fiber | NDF | % | NIR, WC | x | x | x | x |
| Acid Detergent Fiber | ADF | % | NIR, WC | x | x | x | |
| Lignin | % | NIR, WC | x | ||||
| Total Digestible Nutrients | TDN | % | NIR | x | x | x | x |
| Net Energy of Lactation | NEl | Mcal/lb | NIR | x | x | x | |
| Net Energy of Maintenance | NEm | Mcal/lb | NIR | x | x | x | |
| Net Energy of Gain | NEg | Mcal/lb | NIR | x | x | x | |
| Metabolizable Energy | ME | kcal/lb | NIR | x | x | x | |
| Moisture | % | Oven | |||||
| Dry Matter4 | DM | % | Oven | x | |||
| Mineral Analyses | |||||||
| Phosphorus | P | % | WC, ICP | x | x | ||
| Potassium | K | % | WC, ICP | x | x | ||
| Calcium | Ca | % | WC, ICP | x | x | ||
| Magnesium | Mg | % | WC, ICP | x | x | ||
| Manganese | Mn | PPM | WC, ICP | x | x | ||
| Iron | Fe | PPM | WC, ICP | x | x | ||
| Aluminum | Al | PPM | WC, ICP | x | x | ||
| Copper | Cu | PPM | WC, ICP | x | x | ||
| Zinc | Zn | PPM | WC, ICP | x | x | ||
| Sodum | Na | PPM | WC, ICP | x | x | ||
| Other Analyses | |||||||
| Total Fat | % | WC | x | x | |||
| Nitrates5 | NO3-N | PPM | WC | x | x | ||
| Ash | % | Oven | x | ||||
| Sulfur | S | % | WC, ICP | x | x | ||
| Arsenic | As | PPM | WC, ICP | x | |||
| Selenium | Se | PPM | WC, ICP | x | x | ||
| Bound Protein | % | NIR | x | ||||
| pH | unitless | WC | x | ||||
| Salt | % | WC | x | ||||
| Total Aflatoxin4 | ppb | WC | x | x | |||
| 2 An index (unitless) most commonly used for forage categorization and marketing. 3 A term that is now obsolete, with the exception that many states still mandate its listing on the label of commercial feedstuffs. 4 When comparing forage lots, feed tags, and/or labels, balancing rations, or conducting cost assessments of forages and all feedstuffs, it is important to use values corrected for moisture (i.e., on a DM basis). 5 Anti-quality factor assessed when conditions for toxic concentrations of the compound are suspected. |
|||||||
Evaluate Forage on More than Just Crude Protein
Most of the classes of livestock that are being fed in the Southeast have relatively low requirements for CP. For example, the CP requirement for beef cows peaks during early lactation at 12% CP and declines to about 7% CP for dry cows. Most of the forages produced in the Southeast can meet these requirements (see inset, “Forage Quality of Major Southern Forages: Summary Statistics”).
Unfortunately, there is a false perception that protein is the most limiting nutrient in the animal's diet. The reality is that the energy value of the forage is usually the most limiting factor in meeting a livestock class's requirements. As a result, many mistakenly believe that CP is the ultimate measure of a forage crop's quality.
Using CP as the sole measure of forage quality can be deceiving. Crude protein, as the name implies, is a crude method for measuring protein. In fact, CP is merely an estimate of nitrogen content (N, % x 6.25 = CP, %) and must be considered in context of plant maturity, species, fertilization rate, and many other characteristics. For example, a high nitrate concentration in the forage would be measured in the total N fraction, but nitrates are non-protein N.
Certainly, CP is an important indicator of the protein content of a forage crop. However, focusing on CP may cause one to fail to place enough emphasis on meeting energy requirements. Instead of focusing on CP, one should focus first on the amount of digestible energy in the forage.
Near Infrared Spectroscopy
The multiple steps and chemicals involved in the stepwise, “wet chemistry” extraction procedures are dangerous to laboratory workers, time consuming, and expensive. Consequently, forage researchers and nutritionists have developed alternative analysis techniques to mitigate these issues. In the early 1980s, scientists began measuring near infrared reflectance of known forage samples and found good relationships between the reflectance data and many of the forage quality metrics. Near infrared light in the 1100 to 2500 nm wavelength bands reflects in a known and repeatable way when it contacts compounds that contain hydrogen bonds to carbon, nitrogen, and oxygen. Consequently, complex carbon- and nitrogen-containing compounds (e.g., NDF, ADF, lignin, CP, etc.) can be accurately and precisely estimated by measuring the near infrared reflectance spectra. Using these relationships, researchers and engineers developed near infrared reflectance spectroscopy (NIRS) equipment and software that provide a safe, time-efficient, and cost-effective alternative to wet chemistry extraction methods (Figure 5).
 Figure 5. Analyses that once took numerous hours in the laboratory can now be performed in seconds using near infrared (NIR) spectroscopy. Though wet chemistry methods are still performed to check the calibration of the NIR system, the main forage quality measurements can now be accurately made at relatively low cost.
Figure 5. Analyses that once took numerous hours in the laboratory can now be performed in seconds using near infrared (NIR) spectroscopy. Though wet chemistry methods are still performed to check the calibration of the NIR system, the main forage quality measurements can now be accurately made at relatively low cost.Additionally, the NIRS technology does not cause a sample to be destroyed. This enables researchers at multiple laboratories to analyze the exact same sample. Consequently, researchers can develop, refine, and verify calibration equations for new forage species or new metrics of nutritive value. For example, the NIRS system enables laboratory technicians to identify outliers (samples that do not fit the calibration well), which can be analyzed with wet chemistry and included in the calibration equation to make the equation more robust. In this same way, the NIRS Forage and Feed Testing Consortium continually refines a number of standardized equations and provides them to forage and feed testing laboratories. The non-destructive nature of NIRS also allows laboratories to use standard samples to conduct quality assurance procedures, which ensures accuracy and precision in their results. The National Forage Testing Association (NFTA) coordinates a certification process that conducts random tests of their member laboratories. They send a known sample to the participating lab and grade the lab's results. In this way, the advent of NIRS has substantially improved the quality and consistency of forage analysis results.
Simplifying Forage Quality Assessments
Animal performance (whether defined as the production of meat, milk, fiber, or work, or merely the maintenance of body weight and condition) is driven by the number of calories the animal consumes. Though protein, minerals, vitamins, and water must also meet or exceed the requirements for the desired level of performance, the most limiting factor is the amount of digestible energy that the animal consumes.
As a result, a high-quality forage can be defined as one that contains large concentrations of digestible energy and is capable of being consumed in large amounts. Scientists have developed several different measures of forage quality. However, the majority of those forage quality metrics do not easily allow for a comparison of different forage types or species.
To simplify assessments of forage quality, Drs. John E. Moore (University of Florida) and Dan Undersander (University of Wisconsin) developed the Relative Forage Quality (RFQ) calculation. There are two factors used in the RFQ measurement (Equation 1). These include: 1) Total Digestible Nutrients (TDN), which is a measure of digestible energy and 2) a calculated prediction of dry matter intake (DMI).
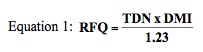
Nutritionists know that TDN and digestible energy (DE) can be thought of as synonyms and used interchangeably, since TDN multiplied by 4.409 equals the DE in Mcal/kg. Additionally, animal scientists have conducted numerous feeding studies and continue to refine prediction equations of DMI for many forage species. Individually, the factors of TDN and DMI can fairly represent elements of forage quality for a particular forage species and type. However, when combined, these two factors provide a robust measure of forage quality. For a more indepth definition of RFQ and the determination of TDN and DMI, see UGA Extension Bulletin 1367, “Common Terms Used in Animal Feeding and Nutrition.”
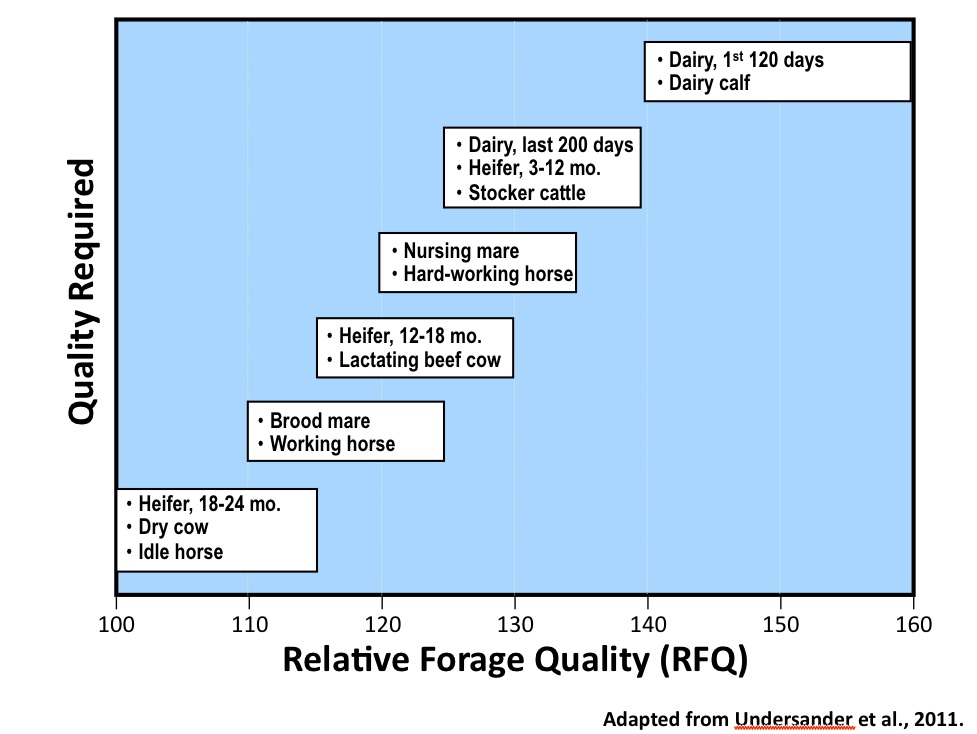 Figure 6. The Relative Forage Quality (RFQ) ranges that are suitable to various livestock classes. Adapted from Undersander et al., 2011.
Figure 6. The Relative Forage Quality (RFQ) ranges that are suitable to various livestock classes. Adapted from Undersander et al., 2011.As a result of the robustness of the RFQ measure, scientists have been able to link ranges of RFQ that are most likely to meet the needs of different animal classes. These ranges can be found in Figure 6 and would allow the livestock manager who has RFQ data on a particular lot of forage to quickly determine if it is appropriate to the needs of the animal class being managed.
These ranges illustrate the RFQ values that are most likely to minimize supplementation. Just because a forage lot falls within these recommended ranges DOES NOT mean that it will automatically provide all the nutrients needed for the livestock being fed. One does not use RFQ to develop a ration. However, RFQ provides a reasonable first approximation as to whether or not a forage will provide a cost-effective base to the diet being fed to the selected animal class.
An RFQ value that is lower than the identified range could still work for the animal class that is being fed. However, additional supplementation will likely be required. This additional supplementation may make the ration (forage + supplement) less economical.
RFQ is a Better Equalizer
Consider the following, real-world example. Pictured in Figure 7 are 25-lb piles of choice alfalfa and standard bermudagrass that were freshly cut from plots at the UGA Plant Science Farm in Athens, Ga. Selected measures of forage quality for these two piles of fresh forage are listed in Table 3, along with measurements of the size and volume of each pile.
 Figure 7. A 25-lb pile of alfalfa (L) and bermudagrass (R) that had been freshly cut with a flail plot harvester. The loose pile (no compression) illustrates the difference in volume each required.
Figure 7. A 25-lb pile of alfalfa (L) and bermudagrass (R) that had been freshly cut with a flail plot harvester. The loose pile (no compression) illustrates the difference in volume each required.Note that despite having the exact same weight, the loose pile of alfalfa is shorter and narrower than that of the bermudagrass. Consequently, it has a smaller volume. The forage quality analysis indicates similar levels of TDN. However, the RFQ of the alfalfa is substantially higher. This is because the DMI predicted for these forage lots differs substantially. If one were to feed forage from these two lots ad libitum (free choice) with no additional supplementation to dairy cows, for example, it is estimated that the cows fed the choice alfalfa would consume 70 lbs more forage per 1000 lbs of b.w. relative to the cows fed the standard bermudagrass. Consequently, those dairy cows on the alfalfa would have consumed ~24% more TDN than those fed the bermudagrass.
Table 3. An illustration of the combination of energy concentration and the importance of supporting high DM intake (DMI). Note that despite similar TDN values, the higher DMI of the alfalfa predicts much higher TDN intake.
| Item | Units | Alfalfa | Bermuda |
| Weight | lbs | 25.0 | 25.0 |
| Loose Pile Height | in. | 22.5 | 25.5 |
| Loose Pile Diam. | in. | 44.3 | 60.0 |
| Approx. Volume | in.3 | 12,000 | 24,000 |
| RFQ | 144.7 | 110.4 | |
| TDN | % | 60.2 | 59.6 |
| DMI | % of b.w. | 3.0 | 2.3 |
| TDN Intake | lbs per 1000 lbs b.w. |
17.8 | 13.6 |
This example illustrates that RFQ is a more robust and superior measure of forage quality than other single measurements. By combining TDN and DMI, the RFQ index provides a better indication of overall forage quality.
Reading a Forage Quality Analysis
The results of a forage quality analysis performed at the University of Georgia's Feed and Environmental Water Laboratory are presented in a report (see inset, “Highlights of a Forage Quality Analysis Report”). The results of a forage quality analysis are reported on an “As-Sampled” and on a “Dry-Matter” basis. Other laboratories may report “As-Sampled” basis values as “As Fed,” “As Received,” or similar. In each case, their meaning is that the percentages and concentrations are not on a dry-matter basis. In nearly all situations, however, the values in the “Dry-Matter” basis column are the most useful for comparisons among forage lots, ration balancing, and assessments of economic value.
In addition to the items highlighted in the inset, care should be taken in interpreting the values in the context of the forage crop species and livestock species/class being fed. A common example is that TDN values from a forage lot that is analyzed in the context of beef or dairy cattle feed will be approximately 20 percentage points higher than the same lot analyzed as a feedstock for horses. The reason for this is the inherent differences in gastrointestinal physiology of these herbivorous species (pre-gastric vs. hind-gut fermenter) and not because of differences in the forage quality.
Management Factors Affect Forage Quality
As implied in the previous sections, implementing proper management is critical to producing and harvesting high-quality forage. The most critical management factors and their relative importance with regard to forage quality are listed in Table 4. Certainly, there are many additional factors that affect forage quality. However, following the recommendations for each of these factors will enable one to produce and utilize forage that optimizes quality and nutrient yield.
Table 4. The relative importance of the primary factors that affect the nutritive quality of forage and general recommendations on best management practices that optimize quality.
| Importance | Factor | Recommendations |
| High | Forage Maturity | Cut the forage in the late vegetative or early reproductive stages of growth. See the harvest recommendations in Table 5 for detailed information on individual species. |
| High | Forage Species | Use a high-quality forage species that persists and can be produced economically in your environment. Species resistant to drought and temperature extremes should be used. |
| Moderate | Forage Utilization | Grazed forage is generally higher quality than conserved forage (i.e., hay, silage, etc.) because of animal selectivity and because fresh forage is generally higher in digestible nutrients. However, selectivity may reduce overall forage utilization compared to mechanically harvested systems. |
| Moderate | Variety | Use varieties that have proven to provide a good balance of high quality and high yields. Select disease- and insect-resistant varieties. |
| Moderate | Storage | Protect hay bales from rainfall and weathering during storage (e.g., barn, tarp, etc.). Properly pack and exclude oxygen from forage that is being ensiled. |
| Moderate | Rain Damage | Avoid cutting if significant rainfall (> 0.50 inches) is predicted during curing, but take care to avoid allowing forage to become overly mature. |
| Moderate | Heat Damage | Dry forage to the appropriate moisture for making hay (Round: 15%; Square: 18%) and store in a manner that allows adequate ventilation. Maintain integrity of oxygen barrier in silage storage. |
| Low | Fertilization | Fertilize based on soil test recommendations and at recommended times to sustain CP/mineral concentrations in the forage and to maximize vegetative mass in the standing forage. |
Highlights of a Forage Quality Analysis Report
There is a lot of useful information on a forage analysis report. However, it can be overwhelming. Highlighted below are the five key aspects of a forage analysis report. In nearly all situations, focus should be placed on the values in the “Dry-Matter” basis column. Because moisture can vary across a wide range, using the DM basis will allow for more of an “apples-to-apples” comparison. Furthermore, the DM percentages and concentrations are the values used by most nutritionists when developing rations and determining the economic value of a forage lot.

Forage Maturity
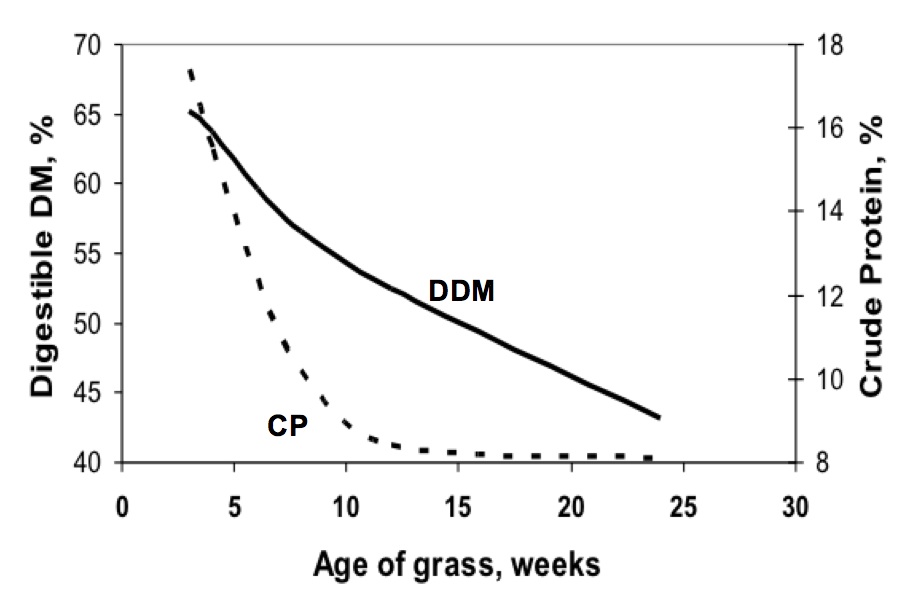 Figure 8. The digestible dry matter (DDM) and crude protein (CP) of 'Coastal' bermudagrass as affected by plant maturity in south Georgia. Source: Burton et al., 1963. Agron. J. Coastal Plain Experiment Station, Tifton, Ga.
Figure 8. The digestible dry matter (DDM) and crude protein (CP) of 'Coastal' bermudagrass as affected by plant maturity in south Georgia. Source: Burton et al., 1963. Agron. J. Coastal Plain Experiment Station, Tifton, Ga.Maturity is the most important factor affecting forage quality. Young, leafy vegetative growth has a higher level of digestible nutrients and protein, which declines as the plants progress toward maturity (Figure 8). Older forage has fewer leaves, more stems, and a higher fiber (NDF) content. As plants mature, more lignin is deposited. Lignin gives the plant strength and rigidity. Lignin also is a natural chemical barrier that plants use to protect themselves from attacks from bacteria, fungi, and insects. The mechanism that the plant uses to provide this protection also means the forage is protected against digestion. Therefore, lignin causes the forage to be much less digestible and less capable of providing the energy needs of the animal. Even though more total DM yield accumulates with advancing forage maturity from vegetative to reproductive stage of growth, there is a point where the amount of digestible dry matter harvested per acre (digestible yield) no longer increases. Figure 9 highlights this phenomenon in bermudagrass, but all forage crops exhibit this same relationship.
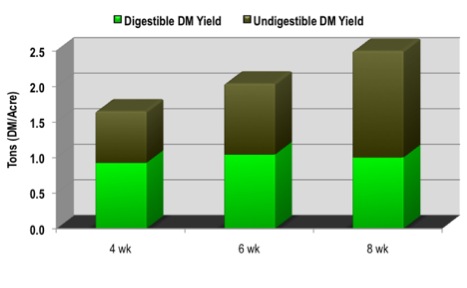 Figure 9. As an illustration of a typical situation, the total yield of bermudagrass increases with maturity, but the amount of digestible dry matter (DM)/acre does not generally increase beyond four-week-old growth. Because of increasing fiber and lignin concentrations, more undigestible DM is produced and lowers the quality.
Figure 9. As an illustration of a typical situation, the total yield of bermudagrass increases with maturity, but the amount of digestible dry matter (DM)/acre does not generally increase beyond four-week-old growth. Because of increasing fiber and lignin concentrations, more undigestible DM is produced and lowers the quality.Because of the effects of advancing maturity on quality, it is critical to harvest the crop whenever the forage reaches the recommended stage for harvest. Table 5 lists the maturity stages that should be targeted for some of the major forage crops. Delaying a harvest beyond the recommended maturity stage will result in forage that is less digestible and much less capable of being consumed at a high rate of intake. Harvesting slightly earlier than the recommended maturity is an option and may be advisable to avoid weather-related risk.
Table 5. Harvest recommendations for some of the major hay crops.
| Harvest Recommendations | |||
| Hay Crop | First Harvest | Subsequent Cutting | Special Considerations |
| Alfalfa* | Late bud stage | Early bloom (usually after every 28-32 days). | In the spring after establishment, allow the first cutting to reach mid-bloom. |
| Annual Ryegrass | Boot stage | When regrowth reaches 10-12 in. (if applicable) | Harvest if forage growth ceases because of hot or dry weather. |
| Bermudagrass | 12 - 16 inches | 3.5 - 5 week intervals | If the variety rarely gets taller than 14 - 15 inches, take the first harvest at 12 inches. |
| Orchardgrass | Boot - early head | 4 - 6 week intervals | Harvest if forage growth ceases because of hot or dry weather. |
| Red or Ladino Clover | Early Bloom | Early Bloom | When grown with a grass, cut at the correct stage for the grass. |
| Small Grains | Boot - early head | N/A | If the boot-early head stage is missed, take the first harvest at the dough stage. |
| Tall Fescue | Boot - early head | 4 - 6 week intervals | Harvest if forage growth ceases because of hot or dry weather. |
| Winter Annual Legume | Early Bloom | N/A | When grown with a grass, cut at the correct stage for the grass. |
| * These recommendations aid the longevity of the alfalfa stand in the South and may not be appropriate for other areas in the U.S., especially when extremely high quality is desired. | |||
Forage Species Differ in Quality
Comparing the forage quality of one lot to another lot from the same crop species can be done simply by simultaneously comparing the TDN, CP, ME, and other measures previously discussed. However, comparing forage from one species to another is more difficult.
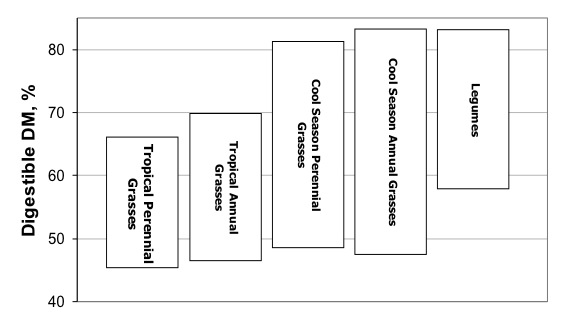 Figure 10. Digestibility ranges of major forage types. Note that the ranges overlap, but some forage types are more likely to be lower in quality than others.
Figure 10. Digestibility ranges of major forage types. Note that the ranges overlap, but some forage types are more likely to be lower in quality than others.
It is well known that different forage types exhibit differences in digestibility and nutritive value (Figure 10). In general, grasses have much higher NDF than legumes. As a result, legumes are generally more digestible than grasses. Similarly, cool season grasses are typically lower in NDF and more digestible the warm season grasses. However, the digestibility of NDF differs between these forage types. For example, a cool season grass with 60% NDF may actually be less digestible than a warm season grass with 60% NDF. This is because of differences in the type of fiber and lignin created by these forage types and species. Even within a forage species, differences are sometimes found. For example, there are substantial differences in digestibility among some bermudagrass varieties (see inset, “Digestibility Sometimes Differs Between Varieties”).
Forage Quality of Major Southern Forages: Summary Statistics
Figure 11 provides a graphical summary of the statistics on more than 16,000 forage samples that were submitted to the University of Georgia's Feed and Environmental Water Lab between July 2003 and February 2011. To better understand how a particular forage lot compares to others, compare the data on the report to the summary statistics provided here.
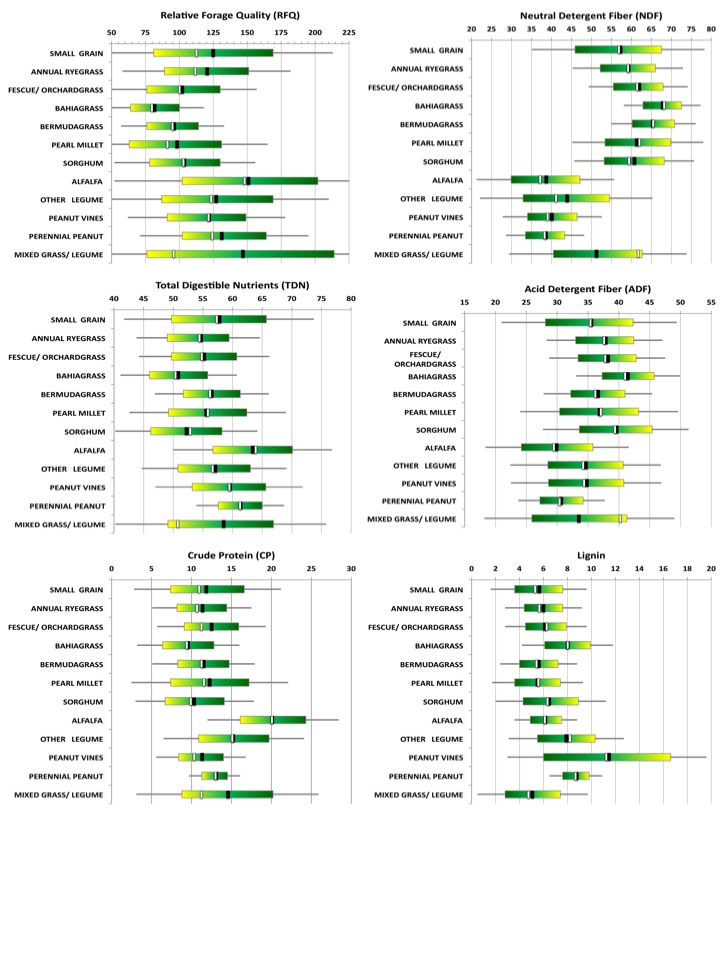 Figure 11. The average (black vertical lines), median (white vertical lines), typical expected6 range (color bars), and the extent7 of what is commonly low or high for a species (extent of horizontal gray lines) for RFQ, TDN, CP, NDF, ADF, and lignin in samples of various forage species submitted to the UGA Feed and Environmental Water Laboratory from July 2003 - February 2011.
Figure 11. The average (black vertical lines), median (white vertical lines), typical expected6 range (color bars), and the extent7 of what is commonly low or high for a species (extent of horizontal gray lines) for RFQ, TDN, CP, NDF, ADF, and lignin in samples of various forage species submitted to the UGA Feed and Environmental Water Laboratory from July 2003 - February 2011.6 One standard deviation about the mean. 7 Two standard deviations about the mean.
Digestibility Sometimes Differs Between Varieties
In general, digestibility decreases as the fiber content (NDF) increases. However, NOT ALL FIBER IS CREATED EQUAL. Differences in NDF values alone do not always correspond to similar differences in digestibility within a species. This is best illustrated by examining the differences between 'Coastal' bermudagrass and 'Tifton 85' bermudagrass. Studies performed at the Coastal Plain Experiment Station in Tifton, Ga., found that despite a higher NDF concentration, 'Tifton 85' bermudagrass provided more animal gains. An examination of DM digestibility showed that the 'Tifton 85' was more digestible than 'Coastal,' despite having higher NDF (Table 6). When the digestibility of the NDF was examined, it confirmed that the NDF produced by the 'Tifton 85' was more easily digested. Further examination showed that this was because the type of lignin in 'Tifton 85' is degraded more easily than the lignin in 'Coastal.'
Table 6. Differences in neutral detergent fiber (NDF) concentration, actual digestibility, and NDF digestibility of 'Coastal' and 'Tifton 85' bermudagrass at two different maturities. Adapted from: Mandebvu et al., 1999. J. Ani. Sci. 77:1572-1586.
| Variety | Maturity | NDF | DM Digestibility48h 1 | NDF Digestibility48h 2 |
| -------------- % of dry matter -------------- | ||||
| Coastal | 3-wk | 66.9 | 51.4 | 42.6 |
| 6-wk | 68.9 | 50.8 | 41.0 | |
| Tifton 85 | 3-wk | 68.6 | 61.7 | 60.6 |
| 6-wk | 72.3 | 56.9 | 55.6 | |
| 1 In vito dry matter digestibility (IVDMD) during a 48 h period. 2 NDF digestibility during a 48 h period. |
||||
Summary
Many forage buyers and sellers judge and appraise the value of a hay crop based on feel, texture, smell, or appearance. Attempting to assess forage quality in this way will likely lead to erroneous and uneconomical purchasing and feeding decisions. Evaluating forages for nutritive value allows the producer/manager to more accurately appraise and market available forage lots, develop a balanced ration, and use forages more cost-effectively in feeding programs.
Modern forage quality determinations can be done quickly and cost-effectively. In addition, nutritionists have developed the Relative Forage Quality (RFQ) index to be an easy-to-use tool for comparing forage lots. With the development of RFQ, producers/managers can make comparisons among forage lots from widely different species and determine if the lots are appropriate to the livestock class being fed. Once this is determined, the usefulness and economic value of the forage lot can be refined through ration development using other aspects of forage quality, such as TDN, metabolizable and net energy, CP, etc.
Managers should also be aware of the influence of management on forage quality. Key factors, such as maturity of the crop at harvest and the forage species, should be focal points.
Using tabular data out of a nutritional guide can cause one to over- or underestimate the nutritive value of a forage lot. Long-term averages, such as those provided in Figure 11, provide a benchmark by which to judge a given lot of forage. As illustrated in Figure 11, however, the actual results will vary, in some cases considerably.
The only way to know what the nutritive value and quality of the lot of forage one is dealing with is to conduct a forage test. By measuring, monitoring, and managing forage quality and adjusting the ration accordingly, producers can keep animal production costs low and increase profitability.
Related Publications and Resources
UGA Extension Bulletin 1373, “Cutting Costs, Not Corners: Managing Cattle in Tough Times.”
UGA Extension Bulletin 1367, “Common Terms Used in Animal Feeding and Nutrition.”
UGA Extension Special Bulletin 58, “Measuring the Dry Matter Content of Feeds.”
UGA Extension Bulletin 895, “Mineral Supplements for Beef Cattle.”
UGA Extension Bulletin 1371, “UGA Basic Balancer Spreadsheet.”
UGA Extension Bulletin 1377, “UGA Feed Cost Analyzer.”
UGA Crop and Soil Sciences CSS-F048, “Using Relative Forage Quality to Categorize Hay.” http://www.caes.uga.edu/commodities/fieldcrops/forages/pubs/RFQcategorization.pdf.
Status and Revision History
Published on Jan 21, 2014
Published with Full Review on Mar 28, 2017
